This gene symbol was allocated by Ausemus et al. (1946) to genes in Marquillo but the designation was later abandoned because no single line possessing either gene could be identified (Knott, 1989).
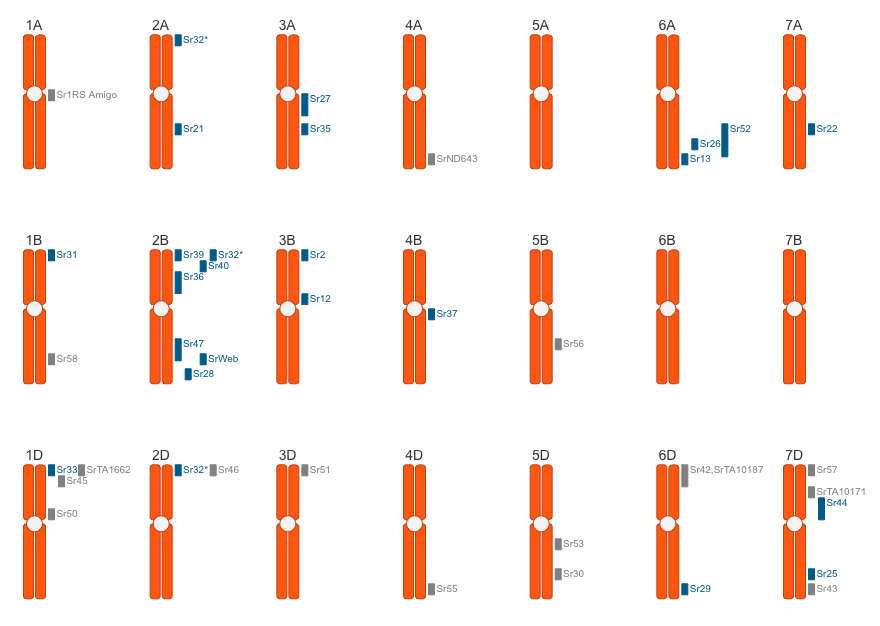
The stem rust resistance genes map is provided by Matt Rouse (USDA-ARS Cereal Disease Laboratory) and Hugues Barbier (Cornell University). Individual loci link to the book “Wheat Rusts: An Atlas of Resistance Genes” by Robert McIntosh, Colin Wellings and Robert Park.
The stem rust resistance genes linked below contain text from “Wheat Rusts: An Atlas of Resistance Genes” by Robert McIntosh, Colin Wellings and Robert Park. Originally published by CSIRO, this electronic copy is offered with their full permission
Sr2
This gene for adult plant resistance shows recessive inheritance.
Chromosome Location
3BS (Hare and McIntosh, 1979). Sr2 is closely associated with Lr27, one of two complementary genes involved in conferring resistance to leaf rust (Singh and McIntosh, 1984a) and with pseudo-black chaff which involves melanin pigmentation of the glumes and stem, particularly below the uppermost node.
Low Infection Type
Usually characterised by slow rust development and low terminal rust responses on field-grown adult plants. More severe rusting may occur above the nodal regions and on the spike.
Environmental Variability
May be less effective under wet and overcast conditions.
Origin
T. turgidum var. dicoccum cv. Yaroslav. Resistance was transferred to Hope and H44-24 by McFadden (1930).
Pathogenic Variability
Virulence is not common and would be difficult to detect on field-grown adult plants. Knott (1977) was unable to detect resistance in lines with Sr2 using North American race 15B-1.
Reference Stocks
s: Chinese Spring*6/Ciano 3B (Singh and McIntosh, 1984a); Chinese Spring*6/Ciano 5B (Singh and McIntosh, 1984a); Chinese Spring*6/Hope 3B (Hare and McIntosh, 1979).
v: Newthatch Sr5 Sr7b Sr12 Sr17. Hope Sr7b Sr9d Sr17; H44-24 Sr7b Sr9d Sr17 (Hare and McIntosh, 1979).
Source Stocks
Sr2 occurs in many wheats developed in areas where stem rust has been a problem. These cultivars were developed in Australia, Canada, Kenya, USA, Mexico and the Indian subcontinent (see Luig, 1983; Roelfs, 1988).
Australia: Songlen Sr5 Sr6 Sr8a Sr36 (Luig, 1983). Warigo Sr7b Sr17 (Hare and McIntosh, 1979). Suneca Sr8a Sr17 (Gyarfas, 1983). Hopps Sr9d (Hare and McIntosh, 1979). Hofed Sr17 (Luig, 1983).
Canada: Pembina Sr5 Sr6 Sr12. Rescue Sr5 Sr9g. Selkirk Sr6 Sr7b Sr9d Sr17 (Hare and McIntosh, 1979). Redman Sr7b Sr9d Sr17; Regent Sr7b Sr9d Sr17 (Hare and McIntosh, 1979); Renown Sr7b Sr9d Sr17 (Luig, 1983).
CIMMYT: Present in many CIMMYT selections. Bluebird series, for example Nuri 70 Sr5 Sr6 Sr8a. Lerma Rojo 64 Sr6 Sr7b Sr9e. Pavon Sr8a Sr9g Sr30 (McIntosh, 1988b).
Indian Subcontinent: Sonalika (McIntosh, 1988b).
Kenya: Kenya Plume Sr5 Sr6 Sr7a Sr8a Sr12 Sr17 (Singh and McIntosh, 1986b). Kenya Page Sr7b Sr17.
USA: Eagle (USA); Kaw. Arthur Sr5 Sr8a Sr36; Arthur 71 Sr5 Sr8a Sr36. Ottawa Sr9d. Lancer Sr9d Sr17; Scout Sr9d Sr17. Karl Sr9d Sr24. Scoutland Sr17.
Use in Agriculture
Sr2 is arguably the most important gene for stem rust resistance and one of the most important disease resistance genes to be deployed in modern plant breeding (McIntosh, 1988b; Rajaram et al., 1988; Roelfs, 1988). With the possible exception of Canada, Sr2 has provided durable resistance since its introduction to hexaploid wheat in the 1920s (McFadden, 1930). In the North American epidemics of the 1950s cultivars such as Regent, Renown and Redman became moderately susceptible. Many wheats with Sr2, including Hope, possess additional genes for resistance such as Sr7b, S19d and Sr17. When the latter genes were effective, they conferred very high levels of resistance. However, the presence of virulence for Sr7b, Sr9d and Sr17 often revealed that the residual resistance conferred by Sr2 was much less effective.
In these situations, Sr2 was overlooked because of the apparent increased disease levels. Hare and McIntosh (1979) described phenotypic effects of Sr2 suggestive of attributes often associated with non-hypersensitive and/or non-specific resistances, thus supporting the conclusion that Sr2 was a source of durable resistance.
The degree of interaction involving Sr2 and other genes is unclear. The high degree of seedling resistance of Hope and several other wheats (IT to North American race 56 was apparently caused by the interaction of Sr2 and Sr9d (Knott, 1968). When present alone, the latter gene confers IT 11+ (Knott, 1968; 1990). The association of Sr2 and distinctive stem and spike blackening (pseudo-black chaff) is well known (see Hare and McIntosh, 1979). Brown (1993) has shown a close association between Sr2 and a seedling leaf chlorosis that is evident at greenhouse temperatures above 22°C.
Sr4
This gene symbol was allocated by Ausemus et al. (1946) to genes in Marquillo but the designation was later abandoned because no single line possessing either gene could be identified (Knott, 1989).
Sr5
(Ausemus et al., 1946) (Plate 3-2)
Synonym
Srrl 1 (Rondon et al., 1966).
Chromosome Location
6D (Sears et al., 1957); 6DS (RA McIntosh, unpublished 1984).
Low Infection Type
Usually 0 to ;. Sr5 is often cited as the ‘immunity’ gene from wheat stem rust research. The low infection type is usually very low but can be significantly higher (IT X) when Sr5 is transferred to a highly susceptible genetic background (Luig and Rajaram, 1972).
Environmental Variability
Low (Luig and Rajaram, 1972).
Origin
Common wheat. Sr5 is present in the Stakman et al. (1962) differential, Reliance C.I.7370, having been inherited from Kanred, a Crimean introduction to the USA.
Pathogenic Variability
Virulence occurs in all geographic areas (Luig, 1983; Huerta-Espino, 1992).
Reference Stocks
i: ISr5-Ra C.I.14159 (Loegering and Harmon, 1969); ISr5-Rb C.I.14161 (Loegering and Harmon, 1969); Line B = W2691*7/Reliance (Luig, 1983); Sr5/7*LMPG (Knott, 1990); Thatcher/10*Marquis (Knott, 1965). Although Marquis is susceptible to many pathotypes of economic significance, lines near-isogenic to Marquis may carry the genes Sr7b, Sr18, Sr19 and Sr20, which are present in this cultivar.
s: Chinese Spring*6/Thatcher 6D (Sears et al., 1957); Chinese Spring*7/Marquis 6D (Sheen and Snyder, 1964). The ‘Marquis’ line used by Sheen and Snyder was probably Thatcher or a closely related derivative.
v: Kanred (Sears et al., 1957). Thatcher Sr9g Sr12 Sr16 (Luig, 1983). Reliance Sr16 Sr18 Sr20 (Luig, 1983).
Source Stocks
Many wheats possess Sr5 (see Luig, 1983; McIntosh, 1988a).
Australia: Zenith. Catcher Sr2 Sr6 Sr8a Sr9g Sr12. Bindawarra Sr6 Sr9b Sr15. Aroona Sr8a Sr9b Sr15. Avocet Sr26; Hybrid Titan Sr26.
China: Dong-Fang-Hong 2; Ke-Fang 1. Dong-Xie 3 Sr31.
Europe: Panis; Primus. Hochzücht Sr9g Sr12. Orlando Sr31.
Indian Subcontinent: NP789 Sr11.
North America: Cheyenne; Chisholm; Justin; Winoka. Arthur Sr2 Sr8a Sr36. Fortuna Sr6 Sr7a Sr17. Era Sr6 Sr8a Sr17. Centurk Sr6 Sr8a Sr9a Sr17. Chris Sr7a Sr8a Sr9g Sr12. Oasis Sr8a Sr36. Apex Sr9g Sr12; Kitt Sr9g Sr12. Thatcher Sr9g Sr12 Sr16. Agatha Sr9g Sr12 Sr16 Sr25.
Use in Agriculture
At various times Sr5 has been a useful and very effective source of resistance. However, resistance proved transient in all geographic areas because of selection and rapid increase of virulent pathotypes. The dominance of Thatcher and its derivatives in the northern Great Plains of North America undoubtedly influenced the increase in pathotypes virulent for Sr5.
Sr6
Knott and Anderson, 1956) (Plate 3-3)
Synonym
SrKa1 (Athwal and Watson, 1954).
Chromosome Location
2D (Sears, 1954; Wiggin, 1955; Sears et al., 1957); 2DS (McIntosh and Baker, 1968). Sr6 showed close linkage with Lr2 and Lr15 and linkage of 19-40 cM with gene C for compact spike (McIntosh, 1988a).
Low Infection Type
0; through X to 3+, varying with pathogen culture and temperature. Sr6 may be dominant or recessive depending on pathogen culture, temperature and genetic background.
Environmental Variability
Most effective at temperatures less than 20°C. Becomes ineffective at 24-27°C (Forsyth, 1956; Watson and Luig, 1968).
Origin
Common wheat. Sr6 was identified in McMurachy, a farmer’s selection from Canada, and Red Egyptian which originated from Ethiopia (Knott and Anderson, 1956). Both are club wheats but are genetically distinct. McMurachy was used as a rust resistant parent in North America and Red Egyptian was used in Kenya to produce a number of wheats that were subsequently widely used as parents, for example Kenya 58 in North America and Kenya W743 in Australia.
Pathogenic Variability
When first exploited Sr6 was very effective in most geographical areas. Now virulence occurs in most areas (Luig, 1983; Huerta-Espino, 1992). Progressive increases in virulence for Sr6 were documented by Watson and Luig (1968). Some cultures with intermediate pathogenicities would be classified as virulent in regular surveys.
Reference Stocks
i: ISr6-Ra C.I.14163 (Loegering and Harmon, 1969); Kenya 58/6*Marquis (Green et al., 1960); Kenya 58/10*Marquis (Knott, 1965); Sr6/9*LMPG (Knott, 1990).
s: Chinese Spring*5/Red Egyptian 2D (Sears et al., 1957).
v: McMurachy (Knott and Anderson, 1956; Watson and Luig, 1963). Red Egyptian Sr8a Sr9a (Sears et al., 1957).
Source Stocks
Many wheats carry Sr6 (see Luig, 1983; McIntosh, 1988a).
Africa: Bonza 63 Sr8a Sr9b. Many Kenyan lines (Knott, 1962a; Watson and Luig, 1963).
Australia: Eureka; Wongoondy. Songlen Sr2 Sr5 Sr8a Sr36. Gamut Sr5 Sr8a Sr12; Oxley Sr5 Sr8a Sr12. Bayonet Sr8a. See Luig (1983).
North America: Milam; Shield; Trapper; Travis; Twin. Selkirk Sr2 Sr7b Sr9d Sr17 Sr23. Manitou Sr5 Sr7a Sr9g Sr12. Centurk Sr5 Sr8a Sr9a Sr17. Butte Sr8a Sr9g. Colt Sr8a Sr9a Sr17 Sr24. Onas Sr10; White Federation 54 Sr10. Homestead Sr17.
Use in Agriculture
Sr6 was a very effective source of resistance when first used in Australia (Eureka) and Canada (Selkirk). Sr6 continues to be present in a range of modern cultivars; however, virulence is now widely distributed in most geographic areas (Luig, 1983).
Knott and Anderson (1956) found that Sr6 was dominant when segregating populations were tested with race 56 but was recessive when they were tested with race 15B.
Sr7
Chromosome Location
4A (Sears et al., 1957; Knott, 1959; Loegering and Sears, 1966); 4AL. Sr7 is genetically independent of the centromere (RA McIntosh, unpublished 1973)
Sr7a
(Loegering and Sears, 1966) (Plate 3-4)
Synonym
Sr7 (Knott and Anderson, 1956).
Low Infection Type
1 to 3C (Knott, 1989); yellow chlorosis/necrosis surrounding uredia is common and characteristic.
Environmental Variability
Presumed to be low.
Origin
Common wheat. Sr7a was originally identified in a number of Kenyan wheats (Knott and Anderson, 1956; Knott, 1962a).
Pathogenic Variability
Virulence occurs in most geographic areas (Luig, 1983; Huerta-Espino, 1992). Intermediate low responses are common. In addition, host genetic background probably has a significant influence on response (Roelfs and McVey, 1979).
Reference Stocks
i: Egypt Na 101/6*Marquis; Kenya 117A/6*Marquis (Green et al., 1960); Sr7a/9*LMPG (Knott, 1990); Sr7a/10*Marquis (Knott, 1965).
s: Chinese Spring*7/Kenya Farmer 4A; Chinese Spring*8/Sapporo 4A (Loegering and Sears, 1966).
v: Kenya 117A C.I. 13140 Sr9b (Knott, 1957b). Egypt Na 101 P.I. 139599 Sr9b Sr10 (Knott, 1957a).
Source Stocks
See lists in Luig (1983) and McIntosh (1988a).
Africa: Kentana 52 Sr6. Kenya wheats (Knott, 1957a, 1957b, 1959, 1962a).
Australia: Khapstein Sr2 Sr13 Sr14 (Knott 1962b). Mendos Sr11 Sr17 Sr36. Chris derivative W3747 Sr12.
Canada: Manitou Sr5 Sr6 Sr9g Sr12.
Japan: Sapporo P.I.81790-2 (Knott and Shen, 1961).
USA: Chris Sr5 Sr8a Sr9g Sr12 (Singh and McIntosh, 1987).
Use in Agriculture
Sr7a has not been added purposefully as a means of achieving resistance, and has been found in wheats studied after commercial release. Singh and McIntosh (1986b) reported interactions of Sr7a and Sr12 resulting in significantly higher levels of resistance than that conferred by either gene acting alone.
Sr7b
(Loegering and Sears, 1966) (Plate 3-5)
Low Infection Type
2 to 3–.
Environmental Variability
Low.
Origin
Common wheat; present in the Stakman et al. (1962) differentials Marquis C.I.3641 and Kota C.I.5878.
Pathogenic Variability
Virulence occurs at high frequency in most geographic areas (Luig, 1983). Moderate to high levels of avirulence may be found in parts of southern Africa (Huerta-Espino, 1992), the USA and Australia.
Reference Stocks
i: ISr7b-Ra C.I. 14165 (Loegering and Harmon, 1969).
s: Chinese Spring*6/Hope 4A (Sears et al., 1957).
v: Marquis Sr18 Sr19 Sr20 (Knott, 1965).
Source Stocks
Sr7b is very common and is not fully catalogued in many wheat cultivars [see list in Luig (1983)].
Australia: Spica Sr17.
Canada: Red Bobs Sr10 (Dyck and Green, 1970).
CIMMYT: Kiric 66 Sr6 (McVey and Roelfs, 1975).
Europe: Fertödi 293 (Luig, 1983); Halle 9H39 (Luig, 1983). Roussalka Sr8a (McVey and Roelfs, 1975).
USA: Geneva; Hart; TAM102. Hope Sr2 Sr9d Sr17 (Sears et al., 1957). Caldwell Sr9d Sr10. Marfed Sr10. Nell Sr17 (Wells et al., 1983). Ceres Sr28; Kota Sr28 (McIntosh, 1978). C.I.12632 Sr36.
Use in Agriculture
Sr7b has not been consciously selected as a source of stem rust resistance. Avirulence on plants with Sr7b was probably present when rust research began in Australia in the 1920s. It disappeared with the extinction of pathotypes present at that time, only to reappear in 1968 with putative introductions from southern Africa (Watson and de Sousa, 1983). The current predominant Australian pathotype is avirulent (Park and Wellings, 1992) and Sr7b confers an effective degree of resistance.
Sr8
(Knott and Anderson, 1956)
Chromosome Location
6A (Sears, 1954; Sears et al., 1957); 6AS (McIntosh, 1972). Sr8 is genetically independent of the centromere (McIntosh, 1972).
Sr8a
(Singh and McIntosh, 1986a) (Plate 3-6)
Synonym
Sr8 (Knott and Anderson, 1956).
Low Infection Type
2– to 3.
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
Common wheat. This gene was first described in Red Egyptian (Knott and Anderson, 1956) but subsequent work demonstrated its presence in many European and Mexican lines. It probably became widespread in modern spring wheats through the use of Italian lines and their derivatives in South America and elsewhere.
Pathogenic Variability
P. graminis populations in most geographic regions are polymorphic for pathogenicity on seedlings with Sr8a (Luig, 1983; Huerta-Espino 1992).
Reference Stocks
i: ISr8-Ra C.I.14176 (Loegering and Harmon, 1969); Red Egyptian/10*Marquis (Knott, 1965); Sr8a/9*LMPG (Knott, 1990).
s: Chinese Spring*5/Red Egyptian 6A (Sears et al., 1957).
v: Mentana (Luig and Watson, 1965). Red Egyptian Sr6 Sr9a (Knott and Anderson, 1956).
Source Stocks
Sr8a is a very common gene. Examples from lists in Luig (1983), McIntosh (1988a) and elsewhere include the following.
Australia: Jacup. Songlen Sr2 Sr5 Sr6 Sr36. Hartog = Pavon ‘S’ Sr2 Sr12 Sr30 (Brennan, 1983). Warigal Sr5 Sr9b Sr15. Condor Sr5 Sr12. Egret Sr9b Sr12.
China: An-Hewi II Sr5.
CIMMYT: Nuri 70 Sr2 Sr5 Sr6. Penjamo 62 Sr5 Sr6 Sr9b. Inia 66 Sr9a Sr11.
Europe: Victor 1 Sr5 Sr6. Golden Valley Sr7b; Roussalka Sr7b.
South America: Frontana Sr9b.
USA: Geneva. Arthur Sr2 Sr5 Sr36. Centurk Sr5 Sr6 Sr9a Sr17. Era Sr5 Sr6 Sr17. Chris Sr5 Sr7a Sr8a Sr9g Sr12. Butte Sr6 Sr9g. Olaf Sr9b Sr12. Benhur Sr10.
Use in Agriculture
Despite its high frequency in wheat cultivars, Sr8a probably has limited effects on field responses to stem rust. Wheats with Sr8a may become susceptible even with avirulent pathotypes.
Sr8b
(Singh and McIntosh, 1986a) (Plate 3-7)
Synonym
SrBB (Luig, 1983).
Low Infection Type
X= to X.
Environmental Variability
Moderate, with lower infection types at cooler temperatures (Luig, 1983).
Origin
Common wheat. Sr8b appears to be derived from Barleta which was probably introduced to Argentina from Spain (Luig, 1983).
Pathogenic Variability
Australian populations are differentiated by their pathogenicity on wheats with Sr8b. Virulence was common in North America and South America, Europe and Africa in the early 1970s (Luig, 1983). In the survey of Huerta-Espino (1992) high levels of virulence were found in western Asia and eastern Europe, northern Africa and western Europe and South America. Populations in southern Africa showed a wide range of variability and those in China were generally avirulent.
Reference Stocks
v: Barleta Benvenuto C.I. 14196 (Singh and McIntosh, 1986a).
Source Stocks
Klein Titan (Singh and McIntosh, 1986a). Bezostaya Sr5 (RA McIntosh and AK Khanzada, unpublished 1989). Klein Cometa Sr30 (Singh and McIntosh, 1986a).
Use in Agriculture
Sr8b is a rare gene. Bezostaya is the only non-South American wheat reported to carry this gene. However, the pedigree of Bezostaya includes Klein 33, which was of Argentinian origin. Resistance is effective to avirulent pathotypes in field plots (Singh and McIntosh, 1986a).
Sr9
(Knott and Anderson, 1956)
Chromosome Location
2B (Sears et al., 1957; Knott, 1959; Knott, 1968; Loegering and Harmon, 1969). 2BL (Sears and Loegering, 1968; McIntosh and Baker, 1970b; McIntosh and Luig, 1973a; Williams and Maan, 1973). Sr9 was mapped at 11 (Sears and Loegering, 1968) and 18 (McIntosh and Baker, 1969) map units from the centromere. It is closely linked with Yr7 (McIntosh et al., 1981) and presumably Yr5, and shows recombination of between 0.15 and 0.3 with Lr13 and Lr23 (McIntosh, 1988a).
Sr9a
(Green et al., 1960) (Plate 3 8)
Synonym
Sr9 (Knott and Anderson, 1956).
Low Infection Type
1– to 2, 23.
Environmental Variability
Low (Green et al., 1960; Loegering and Harmon, 1969).
Origin
Common wheat. Sr9a was first reported in Red Egyptian.
Pathogenic Variability
Wheats with Sr9a produce differential seedling responses in North America (Green et al., 1960), central America, Brazil and possibly Kenya (Luig, 1983). Sr9a is ineffective in tests with Australian pathotypes (Green et al., 1960) and on the Indian subcontinent.
Reference Stocks
i: ISr9a-Ra C.I.14169 (Loegering and Harmon, 1969); Red Egyptian/6*Marquis (Green et al., 1960); Red Egyptian/10*Marquis (Knott, 1965); Sr9a/9*LMPG (Knott, 1990).
s: Chinese Spring*4/Red Egyptian 2B (Sears et al., 1957).
v: Red Egyptian C.I. 12345 Sr6 Sr8a (Knott and Anderson, 1956).
Source Stocks
CIMMYT: Lerma Rojo 64 Sr2 Sr6 Sr7b. Inia 66 Sr2 Sr8a Sr11.
Europe: French Peace Sr7a Sr13 (Knott, 1983).
USA: TAM107. Centurk Sr5 Sr6 Sr8a Sr17. Sentinel Sr6 Sr8a Sr17. Colt Sr6 Sr8a Sr17 Sr24.
Use in Agriculture
Not widely used.
Sr9b
(Green et al., 1960) (Plate 3-9)
Synonym
SrKb1 (Athwal and Watson, 1954).
Low Infection Type
1+ to 3. 2 (DR Knott, pers. comm. 1993).
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
Common wheat. Sr9b was first found in certain Kenyan wheats (Knott and Anderson, 1956). Its presence in the Brazilian cultivar Frontana must derive from Fronteira whose pedigree is Polysu/Alfredo Chaves 6 (Zeven and Zeven-Hissink, 1976).
Pathogenic Variability
Wheats with Sr9b produce differential responses to P. graminis in most geographic areas (Luig, 1983). In the survey of Huerta-Espino (1992) frequencies of virulence for Sr9b were generally high.
Reference Stocks
i: Kenya 117A/6*Marquis, W2402 (Green et al., 1960; Watson and Luig, 1963); Kenya 117A/10*Marquis (Knott, 1965): Sr9b/10*LMPG (Knott, 1990).
s: Chinese Spring*7/Kenya Farmer 2B (McIntosh and Luig, 1973a).
v: Gamenya (Luig, 1983).
Source Stocks
Sr9b occurs in many wheats, especially those of Kenyan origin (see Luig, 1983; McIntosh, 1988a).
Africa: Romany Sr5 Sr6 Sr7a Sr30. Bonza 63 Sr6 Sr8a. Kenya 117A Sr7a Sr10. Kenya Farmer Sr7a Sr10 Sr11. Kenya W744 Sr15.
Australia: Gamenya. Aroona Sr5 Sr8a Sr15; Warigal Sr5 Sr8a Sr15. Egret Sr8a Sr12.
CIMMYT: Penjamo 62 Sr5 Sr6 Sr8a. Pitic 62 Sr8a. Nainari 60 Sr11.
South America: Frontana Sr8a; Rio Negro Sr8a.
USA: Atlas 66 Sr10. Lancota Sr10 Sr17.
Use in Agriculture
Sr9b is relatively common in spring wheats because the gene has been effective in many countries. In Frontana and many of its relatives, Sr9b is linked with Lr13. The presence of Sr9b in some wheats probably resulted from selection for leaf rust resistance.
Sr9c
This symbol was originally reserved for a gene derived from T. timopheevii and subsequently designated Sr36.
Sr9d
Knott, 1966) (Plate 3-10)
Synonym
Sr1 (Ausemus et al., 1946; Knott, 1966, 1968, 1971).
Low Infection Type
1– to 2+.
Environmental Variability
Probably low.
Origin
T. turgidum. Sr9d was originally transferred to the bread wheats Hope and H-44 from Yaroslav emmer (McFadden, 1930). Sr9d is present in the Stakman et al. (1962) durum differentials Mindum, Arnautka and Spelmar.
Pathogenic Variability
In North America, Sr9d was effective against race 56 but was not effective against race 15B which caused catastrophic epidemics in the early 1950s. In the international survey of Huerta-Espino (1992) virulence levels generally approached 100%, except for Turkey. Sr9d has always been ineffective in Australasia.
Reference Stocks
i: Hope/10*Marquis (Knott, 1968); H-44/10*Marquis (Knott, 1968); IHope 2B-Ra (Loegering and Harmon, 1969); Sr9d/8*LMPG (Knott, 1990).
v: Hope Sr2 Sr7b Sr17.
tv: Arnautka (Roelfs and Martens, 1988); Mindum (Roelfs and Martens, 1988); Spelmar (Roelfs and Martens, 1988).
Source Stocks
Australia: Hopps Sr2 (Sunderwirth and Roelfs, 1980). Lawrence Sr2 Sr7b Sr17. Spica Sr7b Sr17.
Canada: Selkirk Sr2 Sr6 Sr7b Sr17 Sr23. Redman Sr2 Sr7b Sr17; Renown Sr2 Sr7b Sr17.
USA: Shawnee; Sturdy. Scout Sr2 Sr17.
tv: Nugget.
Use in Agriculture
Sr9d was of some use in both common wheats and durums in North America until the outbreaks of 15B in the early 1950s which affected both common and tetraploid wheats.
Sr9e
(McIntosh and Luig, 1973a) (Plate 3-11)
Synonyms
Srv (Smith, 1957), Srd1v (Kenaschuk et al., 1959).
Low Infection Type
1– to 2+.
Environmental Variability
Low.
Origin
T. turgidum. Sr9e occurs in Vernal emmer selected as a P. graminis tritici differential by Stakman et al. (1962). It is also present in some North American durum wheats. McVey (1990) postulated the presence of Sr9e in 174 of 578 spelt wheat accessions.
Pathogenic Variability
Virulence occurs at a high frequency in North America and at relatively low frequencies in other geographic areas (Luig, 1983; Huerta-Espino, 1992).
Reference Stocks
i: Sr9e/7*LMPG (Knott, 1990).
v: Vernstein (Luig and Watson, 1967).
tv: Vernal emmer (Smith, 1957).
Source Stocks
Australia: Sunstar Sr8a Sr12. Combination III Sr36.
South Africa: SST 3R (Sharma and Gill, 1983); SST-16 (Sharma and Gill, 1983); SST 33 (Le Roux and Rijkenberg, 1987b); SST-66 (Le Roux and Rijkenberg, 1987b).
tv: C.I.7778 (Luig and Watson, 1967). Sr9e is present in a range of North American durums (RA McIntosh, unpublished 1973). The Mexican cultivar Yavaros 79 Sr12 and various durums with additional genes were shown to carry Sr9e (Singh et al., 1992).
Use in Agriculture
The use of Sr9e as a source of resistance in South African bread wheats was followed by an increased pathogen virulence frequency. Although virulence for Sr9e was occasionally detected in Australian surveys, no isolate from wheat-growing areas was virulent on seedlings of Sunstar. Many North American durums appear to carry Sr9e as a component of their oligogenic resistances. However, Sr9e is not effective against race 15B which has been important since the 1950s.
Sr9f
(Loegering, 1975)
Synonym
Srpl (Loegering, 1975).
Low Infection Type
;2– (Roelfs and McVey, 1979).
Environmental Variability
Unknown.
Origin
Common wheat cv. Chinese Spring.
Pathogenic Variability
Most P. graminis f. sp. tritici isolates are virulent.
Reference Stocks
v: Chinese Spring; the contrasting line lacking Sr9f is ISr9a-Ra C.I.14169 (Loegering, 1975).
Use in Agriculture
Wheat possesses many resistance genes such as Sr9f that can be detected with laboratory cultures possessing unusual genes for avirulence. Most of these genes are not catalogued and are of little agricultural importance.
Plate
None available.
Sr9g
(McIntosh and Luig, 1973*) (Plate 3-12)
Low Infection Type
2= to 3.
Environmental Variability
Low.
Origin
T. turgidum var. durum, including the Stakman et al. (1962) differentials Acme and Kubanka. Sr9g was transferred from durum cv. Iumillo to Marquillo and Thatcher from which it was introduced into other common wheats.
Pathogenic Variability
Virulence is common. Apart from southern Africa and Australia, avirulence for Sr9g is uncommon (Luig, 1983; Huerta-Espino, 1992).
Reference Stocks
s: Chinese Spring*7/Marquis 2B Sr16 (McIntosh et al., 1981); Chinese Spring*4/Thatcher 2B Sr16 (McIntosh et al., 1981). The ‘Marquis’ accession used by Sheen and Snyder (1964) was probably Thatcher or a closely related line.
v: Thatcher Sr5 Sr12 Sr16 (McIntosh et al., 1981).
tv: Acme (McIntosh et al., 1981); Kubanka (McIntosh et al., 1981). Both Acme and Kubanka have resistance genes additional to Sr9g.
Source Stocks
Australia: Hartog Sr2 Sr8a Sr30. Corella Sr5 (heterogeneous) Sr8a Sr12. Celebration Sr12 Sr16. Eagle Sr26 (Luig, 1983).
Europe: Hochzücht Sr5 Sr12.
North America: Lee Sr11 Sr16. Many Thatcher derivatives carry Sr9g (McIntosh et al., 1981).
tv: Iumillo which possesses additional genes including, presumably, Sr12 (McIntosh et al., 1981).
Use in Agriculture
Sr9g is one of the genes transferred to Marquillo, and subsequently Thatcher, from the durum cv. Iumillo. Although not commonly effective in North America, avirulence for Sr9g is relatively frequent in Australia, southern Africa and India. Because resistance to avirulent cultures is effective, Sr9g is a useful resistance gene in these areas when used in combinations to ensure protection against a wide range of pathotypes. Because Sr9g is closely linked with Yr7 its presence in some instances may have resulted from selection for resistance to stripe rust.
Sr10
(Knott and Anderson, 1956) (Plate 3-13)
Chromosome Location
2B (DR Knott, pers. comm. 1993). Federation possesses a resistance gene, presumably Sr10, in chromosome 2B (RA McIntosh, unpublished 1980).
Low Infection Type
0; iN to 3C.
Environmental Variability
Temperature sensitive; sensitivity appears to vary between pathogen isolates (Green et al., 1960; Roelfs and McVey, 1979). More effective at lower temperatures.
Origin
Common wheat; first documented in a Kenyan source and Egypt Na95, a derivative of Kenyan parents. However, more recent work indicated this gene is present in Australian wheats developed prior to 1900.
Pathogenic Variability
Virulence for Sr10 was frequent in North America (Roelfs and McVey, 1979). Luig (1983) reported that Sr10 differentiated between pathogen isolates in South America, Israel and South Africa. Huerta-Espino (1992) found moderate levels of avirulence among cultures from Ethiopia and Turkey and high levels among those from Pakistan, Nepal and China.
Reference Stocks
i: Egypt Na95/4*Marquis (Green et al., 1960) = W2404 (Luig, 1983); Line F = W2691 + Sr10 (Luig, 1983).
v: Egypt Na95 Sr7a Sr9b (Knott and Anderson, 1956); Kenya 117A Sr7a Sr9b (Knott and Anderson, 1956).
Source Stocks
Africa: Kenya Farmer Sr7a Sr9b Sr11 (Green et al, 1960). Other Kenyan wheats (Knott, 1957a, 1957b, 1962a).
Australia: Federation (AP Roelfs, pers. comm. 1993).
North America: Geneva (Sorrells and Jensen, 1987); Lemhi; McNair 1003; Saluda; Springfield. Red Bobs Sr7b (Dyck and Green, 1970). Caldwell Sr7b Sr9d. Benhur Sr8a. Atlas 66 Sr9b.
Use in Agriculture
Green and Knott (1962) reported that Sr1O conferred adult plant resistance but there are few data to indicate its real value. Roelfs and McVey (1979) noted that this gene was common in spring wheats in western USA. Sr10 was probably transferred by chance through the use of Kenyan material in the CIMMYT program (Knott, 1990).
Sr11
(Knott and Anderson, 1956) (Plate 3-14)
Synonym
Kc2 (Watson and Stewart, 1956). Knott and Anderson (1956) originally asssigned symbols Sr11 and Sr12 assuming that linked complementary genes were involved. It was later shown that abnormal gametic transmission rates were responsible for disturbed genetic ratios obtained in crosses involving Chinese Spring as the susceptible parent (Luig, 1960, 1968; Sears and Loegering, 1961) and Sr11 became the accepted symbol.
Chromosome Location
6B (Plessers, 1954; Sears, 1954; Knott, 1959); 6BL (Sears, 1966). Sr11 was mapped more than 60cM from the 6B centromere and from the awn inhibitor B2 which is near the centromere (Sears, 1966). Genetically, it shows close repulsion linkage with Lr9 derived from T. umbellulatum, but ER Sears (pers. comm. 1966) obtained a rare recombinant with both genes (available as Sydney University accession C66.10). Heyne and Johnston (1954) reported linkage of 23 cM between Sr11 and Lr3, but workers at The University of Sydney failed to obtain recombination between these genes (Luig, 1964). We do not know of any wheat accession that carries Sr11 and an Lr3 resistance allele.
Low Infection Type
; to 2–. Roelfs and McVey (1979) reported infection types of 2 to 2+3– with certain cultures.
Environmental Variability
Low.
Origin
T. turgidum var. durum cv. Gaza. It is assumed that all sources of Sr11 derive from Bobin W39*2/Gaza material originally produced in Australia, but subsequently widely distributed. Watson and Stewart (1956) concluded that Timstein was derived from this material rather than a T. timopheevii cross.
Pathogenic Variability
Variability occurs in all geographic areas. Virulence frequencies are extremely high in Australia (Zwer et al., 1992), South Africa (Le Roux and Rijkenberg, 1987a), Canada (Harder and Dunsmore, 1990) and the USA (Roelfs et al., 1991), but relatively low on the Indian subcontinent and Europe (Luig, 1983). These results were supported by Huerta-Espino (1992) who also reported low to moderate frequencies of virulence in certain regions of South America and North Africa and no virulence in China.
Reference Stocks
i: ISr11-Ra C.I.14171 (Loegering and Harmon, 1969); Lee/10*Marquis (Knott, 1965).
s: Chinese Spring*7/Kenya Farmer 6B (Loegering and Sears, 1966); Chinese Spring*9/Timstein 6B (Sears et al., 1957).
v: Charter W1371 (Luig and Watson, 1965); Gabo W1422 C.I.12795; Timstein C.I.12347 (Knott and Anderson, 1956; Sears et al., 1957); Yalta W1373 (Luig and Watson, 1965). Lee Sr9g Sr16 C.I.12488 (Knott and Anderson, 1956).
Source Stocks
Sr11 is present in a large number of Australian and Kenyan wheats (see McIntosh, 1988a) and CIMMYT cultivars (Roelfs and McVey, 1979).
China: Qing-Chung 5 Sr5 Sr6.
Europe: Flevina.
India: N.P.790 Sr5.
Use in Agriculture
When first exploited in Australia in the mid 1940s Sr11 was widely effective but its widespread use was followed by increased virulence frequencies. This increase was so spectacular that the pathogen population became genetically fixed for the corresponding pathogen gene for virulence. The consequence is that Sr11 can be detected only with avirulent cultures held in the laboratory and its presence or absence is no longer of relevence to Australian wheat breeders. Charter has been used in India to differentiate pathotypes virulent for Sr11; that is, Charter carries a second gene which cannot be detected using Australian isolates (see Luig, 1983).
Sr12
(Sheen and Snyder, 1964) (Plate 3-15)
Sr12 was first used in conjunction with Sr11 to designate complementary genes thought to be present in Gabo, Lee and Timstein (Knott and Anderson, 1956). Luig (1960) and Sears and Loegering (1961) showed that a single gene (Sr11) was involved and that abnormal gametic transmission rates were responsible for the disturbed genetic ratios originally observed by Knott and Anderson (1956). Sheen and Snyder (1964) then used Sr12 to designate a gene in a Thatcher derivative.
Chromosome Location
3B (Sheen and Snyder, 1964; Knott, 1984); 3BS (McIntosh et al., 1980).
Low Infection Type
;, 2, X to 3.
Environmental Variability
Most effective at temperatures below 20°C.
Origin
Sr12 is derived from T. turgidum var. durum cv. Iumillo and was transferred to Marquillo and, eventually, Thatcher as part of the transference of rust resistance from Iumillo to bread wheat (Hayes et al., 1920).
Pathogenic Variability
Virulence is reported to be common. However, RA McIntosh believes this gene is more effective than reported in the literature. Sr12 also shows interactions with other genes, especially the Sr9 alleles, Sr9b and Sr9g.
Reference Stocks
s: Chinese Spring*7/Marquis 3B (Sheen and Snyder, 1964). Chinese Spring*5/Thatcher 3B Sr16 (Sheen and Snyder, 1964).
v: Thatcher Sr5 Sr9g Sr16 (Luig, 1983). Marquillo Sr9g (Knott, 1984).
Source Stocks
Africa: Kenya Plume Sr2 Sr5 Sr6 Sr7a Sr8a Sr9b Sr17 (Singh and McIntosh, 1986b).
Australia: Tincurrin; Windebri. Celebration Sr9g Sr16 (RA McIntosh, unpublished 1980). Tentative results based on multi-pathotype surveys in Australia indicate that Sr12 may be relatively common. Unfortunately, Sr12 is often difficult to distinguish from Sr6 with Australian P. graminis pathotypes and appropriate genetic studies have not been pursued.
North America: Chris Sr5 Sr8a Sr9g (Singh and McIntosh, 1987). Olaf Sr8a Sr9b. Sr12 is likely to occur in Thatcher derivatives.
tv: Yavaros 79 Sr9e (Singh et al., 1992). Other durums (Singh et al., 1992).
Use in Agriculture
Sr12 was highly effective against pre-1950 Australian pathotypes. After 1950 the effectiveness of this gene in wheats such as Windebri and Celebration was reduced but not completely overcome. In wheats combining Sr12 with Sr9b or Sr9g the presence of Sr12 can be recognised through its effects on the expression of the Sr9 alleles in seedlings inoculated with avirulent pathotypes. These interactions also appear to occur in adult plants. One pathotype in the University of Sydney collection is known to be unusually virulent on seedlings of Iumillo durum. This culture (pt. 21-7, 9 [71178]) is potentially virulent on wheats known to carry Sr12. Both the seedling and adult plant resistances of Marquillo are more effective than those of Thatcher and its derivatives. The reasons for this are not fully undetstood but Sr12 appears to be involved (RA McIntosh, unpublished 1980; see also Knott, 1984).
In North America Sr12 was very effective in conferring resistance to race 56. Race 15B is virulent.
Sr13
Chromosome Location
Distally located in 6AL (McIntosh, 1972).
Low Infection Type
1, 11+, 2– to 23–. 2– at 30°C and 2+3 at 18°C (Roelfs and McVey, 1979).
Environmental Variability
Lowest infection types are obtained at temperatures of 20-28°C, that is, the higher temperature range used for seedling tests.
Origin
T. turgidum var. dicoccum cv. Khapli C.I.4013 in the differential set of Stakman et al. (1962). Sr13 was transferred to the common wheat line, Khapstein by WL Waterhouse.
Pathogenic Variability
Virulence for Sr13 appears to be extremely rare except in India and Pakistan (Luig, 1983) where Khapli emmer is cultivated. Occasional cultures from Europe and Africa produced high infection types on Khapstein (Luig, 1983). Knott (1990) reported a virulent culture of North American race 11. Huerta-Espino (1992) detected one isolate with virulence in Ethiopian samples, one in Turkish and two in Spanish collections.
Reference Stocks
i: Khapstein/10*Marquis (Knott, 1965); Sr13/9*LMPG (Knott, 1990).
v: Khapstein Sr2 Sr7a Sr14 (Knott, 1962b).
tv: Khapli emmer Sr7a Sr14.
Source Stocks
Australia: Wialki (Luig, 1983). Madden Sr2 Sr9b Sr11 (Luig, 1983).
Europe: French Peace Sr7a Sr9a (Knott, 1983).
tv: St464. Probably present in some durums especially those derived from St464.
Use in Agriculture
Because of its widespread effectiveness, Sr13 is potentially useful as a source of resistance to stem rust and has been exploited in some Australian wheats. However, RA McIntosh, RG Rees and GJ Platz (unpublished, 1985) showed that wheats with Sr13 expressed comparatively high adult plant reactions to avirulent cultures and experienced grain weight losses of about 40% relative to chemically protected controls. Related lines lacking the Sr13 allele gave much higher losses. The origin of Sr2 (RA McIntosh, unpublished 1980) and Sr7a (Knott, 1962b) in Khapstein must be questioned because neither gene has been reported in Khapli. In his early studies on hexaploid/tetraploid wheat crosses Waterhouse (1933) reported that cv. Steinwedel and its derivatives were the only hexaploids not producing chlorotic (hybrid chlorosis) hybrids with Khapli. Sterility in pentaploids may have led to outcrossing and Sr2 and Sr7a may be derived from other sources. RA McIntosh (unpublished, 1985) showed that a gene (Srdp2) in a Golden Ball-derived hexaploid wheat, accession W3504, was allelic with Sr13.
Sr14
Chromosome Location
1BL (Baker and McIntosh, 1973; McIntosh, 1980). Sr14 is located very close to the centromere.
Low Infection Type
1N to 3C.
Environmental Variability
Lowest responses associated with Sr14 are produced under high temperature and high light conditions (Luig, 1983; Gousseau et al., 1985). These appear to enhance the distinct necrosis which is very characteristic of this gene (Knott, 1989).
Origin
T. turgidum var. dicoccum cv. Khapli. Sr14 and other genes were transferred to the hexaploid cv. Steinwedel, resulting in cv. Khapstein (Waterhouse, 1933).
Pathogenic Variability
Maximum levels of avirulence are produced by relatively few isolates in most geographic areas. Under high temperature and high light conditions slight reductions in infection type and some necrosis can be detected with many pathogen cultures normally considered virulent (RA McIntosh, unpublished 1975). Luig (1983) reported possible instances of avirulence in European, Israeli and South African cultures, whereas Huerta-Espino (1992) observed avirulence in occasional cultures from Ethiopia and Ecuador. In North America avirulence is limited to race 56.
Reference Stocks
i: Khapstein/10*Marquis (Kao and Knott, 1969); Line A = W2691*2/Khapstein (McIntosh, 1980).
v: Khapstein Sr2 Sr7a Sr13 (Knott, 1962b).
tv: Khapli emmer Sr13.
Source Stocks
Possibly present in some durum wheats where it is combined with other genes such as Sr9e, Sr9d, Sr13 or Srdp2. Sr14 is probably present in the USA durum cv. Yuma (AP Roelfs, pers. comm. 1993).
Use in Agriculture
Sr14 has rarely been deployed in commercial wheats.
Sr15
Watson and Luig, 1966) (Plate 3-18)
Sr15 is completely associated with genes Lr20 and Pm1 (Watson and Luig, 1966; McIntosh, 1977), hence the genetics of these factors are relevent to those for Sr15. On the basis of mutation studies, McIntosh (1977) concluded that Sr15 and Lr20 were the same gene.
Chromosome Location
7A (Sears, 1954); 7AL (Sears and Briggle, 1969). Distally located to Sr22 (The and McIntosh, 1975).
Low Infection Type
0; to X++.
Environmental Variability
Temperature sensitive; lowest reactions develop at greenhouse temperatures of 15-18°C. Ineffective at temperatures above 26°C (Gousseau et al., 1985). Roelfs and McVey (1979) list IT ;1+N at 18°C, 3N at 21°C and 44C at 23°C.
Origin
Common wheat. Although Sr15 was first described in cv. Norka, it was known to be present in some unrelated cultivars.
Pathogenic Variability
Pathogenic polymorphisms occur for Sr15 in most geographical areas. However, virulence levels tend to be very high except in Australia, parts of Africa and North America (Luig, 1983; Huerta-Espino, 1992). Pathotypes can be grouped according to the infection types produced on selected genotypes under controlled conditions. At least five levels of pathogen responses can be distinguished among the Australian pathotype collection (Luig, 1983). Pathotypes giving the higher intermediate responses would be classified as virulent in many laboratories, or if temperatures exceeded 18-20°C.
Reference Stocks
s: Chinese Spring*5/Axminster 7A.
v: Line AB = W2691*2/Norka (Luig, 1983); Norka (Watson and Luig, 1966).
Source Stocks
All wheats with Lr20 and Pm1. However, some mutants lacking Pm1 but possessing Lr20 and Sr15 were generated in the mutagenesis study of McIntosh (1977).
Africa: Kenya W744 Sr9b.
Australia: Angas; Aroona; Fedka. Festival Sr9b. Tatiara Sr9b Sr12.
Europe: Anfield; As II; Maris Halberd; Sappo; Timmo.
USA: Wared.
Use in Agriculture
Sr15 gives satisfactory protection to avirulent pathotypes at lower temperatures. However, the high degree of pathogenic variation and temperature sensitivity make it extremely unreliable. At best, it has been used as a fortuitous component of oligogenic resistances.
Sr16
(Loegering and Sears, 1966) (Plate 3-19)
Synonym
Srr12 (Rondon et al., 1966).
Chromosome Location
2B (Sears et al., 1957; Loegering and Sears, 1966); 2BL (Sears and Loegering, 1968). Sr16 is distal to, and genetically independent of, Sr9 (Loegering and Sears, 1966). It is allelic with a gene (SrKt2) in Kota (RA McIntosh, unpublished 1980).
Low Infection Type
2 to 3.
Environmental Variability
No variation was detected by Roelfs and McVey (1979).
Origin
Common wheat cv. Reliance. Sr16 is probably present in Kanred, a Crimean wheat used as one of the parents leading to Reliance and Thatcher.
Pathogenic Variability
Avirulence is uncommon. Huerta-Espino (1992) recorded avirulent cultures among collections from Ethiopia, Kenya, Nepal, Chile and Paraguay. Most isolates of the wheat stem rust are normally considered to be virulent. However, comparison of rust infections on seedlings of ISr16-Ra and Chinese Spring often indicate very slight reductions in uredinial development on the line with Sr16 (RA McIntosh, unpublished 1976).
Reference Stocks
i: ISr16-Ra (Loegering and Harmon, 1969); ITha 2B-Ra (Loegering and Sears, 1973).
s: Chinese Spring*7/Marquis 2B Sr9g (Williams and Kaveh, 1976); Chinese Spring*5/Thatcher 3B Sr12 (Loegering and Sears, 1973); Chinese Spring*4/Thatcher 2B Sr16 (Sears et al., 1957).
v: Reliance Sr5 (Rondon et al., 1966). Thatcher Sr5 Sr9g Sr12 (Loegering and Sears, 1966).
Source Stocks
Because of the high frequencies of virulence in the pathogen, searches for Sr16 are rarely undertaken. Consequently, Sr16 probably occurs in more wheat cultivars than has been reported. It is common in Thatcher derivatives (Luig, 1983), for example Manitou Sr5 Sr6 Sr7a Sr9g Sr12, Neepawa Sr5 Sr7a Sr9g Sr12, Lee Sr9g Sr11, Celebration Sr9g Sr12.
Use in Agriculture
Not intentionally deployed in agriculture.
Sr17
(McIntosh, 1988a) (Plate 3-20)
Synonym
sr17 (McIntosh et al., 1967). Because this gene is recessive, it was originally designated with a lower case letter. However, adoption of the Rules of Genetic Nomenclature for Wheat (McIntosh, 1988a) required that all genes involved in disease response should be designated with upper case letters irrespective of dominance. This decision was taken in order to provide for less ambiguity in verbal communication of genetic information.
Chromosome Location
7B (Law and Wolfe, 1966); 7BL (McIntosh et al., 1967). Sr17 is genetically linked with Pm5 and Lr14a (see McIntosh, 1988a).
Low Infection Type
;, X, X+.
Environmental Variability
Temperature-sensitive (Roelfs and McVey, 1979). Sr17 is more effective at low temperatures, becoming ineffective above 25°C.
Origin
Assumed to be T. turgidum var. dicoccum cv. Yaroslav emmer used in the development of Hope and H-44 by McFadden (1930). Sr17 has not been detected in a tetraploid wheat by formal genetic studies. Indeed an accession of Yaroslav that possesses the relevant genes of Hope wheat, that is, Sr2, Sr17, Lr14a and Pm5, is not available.
Pathogenic Variability
P. graminis populations in North America and Australia are polymorphic (Roelfs and McVey, 1979; Luig, 1983). Huerta-Espino (1992) found low frequencies of avirulence in North Africa and Spain, Kenya and the Malagasy Republic, Turkey and South America.
Reference Stocks
s: Chinese Spring*6/Hope 7B (McIntosh et al., 1967).
v: Hope Sr2 Sr7b Sr9d (McIntosh et al., 1967; Knott, 1971); H-44 Sr2 Sr7b Sr9d (McIntosh et al., 1967; Knott, 1971). Spica Sr7b (McIntosh et al., 1967).
Source Stocks
Sr17 is present in many USA and Mexican wheats (Roelfs and McVey, 1979), particularly those with Lr14a and Pm5 as all three genes are linked. European workers (Heun and Fischbeck, 1987a, 1987b; Hovmøller, 1989; Lutz et al., 1992) reported that the gene Mli for resistance to powdery mildew is identical to Pm5. It would be of interest to determine if wheats reported to possess Mli also carried Sr17 or Lr14a.
Africa: Giza 144 Sr11.
Australia: Glenwari; Hofed. Gala Sr2. Warigo Sr2 Sr7b. Lawrence Sr2 Sr7b Sr9d. Mendos Sr7a Sr11 Sr36. Hopps Sr9d
Canada: Selkirk Sr2 Sr6 Sr7b Sr9d Sr23.
CIMMYT: Nadadores Sr11.
Europe: Sava Sr5. Adam Sr5 Sr8a. Dunav-1 Sr9b.
India: Kalyansona.
New Zealand: Aotea.
USA: Auburn; Brule; Gage; Larned; Riley 67; Scoutland; Winalta. Scout Sr2. Newthatch Sr2 Sr5 Sr7b Sr12. Redman Sr2 Sr7b Sr9d; Renown Sr2 Sr7b Sr9d. Lancer Sr2 Sr9d. Era Sr5 Sr6 Sr8a. Centurk Sr5 Sr6 Sr8a Sr9a. Homestead Sr6. Colt Sr6 Sr8a Sr9a Sr24. Lancota Sr9b Sr10. Osage Sr24.
Use in Agriculture
From the time of their release in the 1920s, Hope and H-44 have displayed durable resistance to stem rust. A vast literature on these resistances indicates involvement of genes conferring both seedling and adult plant resistances. At various times, and using a limited range of pathogenic variability, researchers have obtained different results when determining the resistance genotype [see Hare and McIntosh (1979) for review]. Sr17 is a significant component of this resistance and can be found in a wide range of Australian, Mexican, USA, Canadian and Indian cultivars. However, the durability of resistance in Hope and H-44 and their derivatives is associated with Sr2.
Sr18
(Baker et al., 1970) (Plate 3-21)
Synonyms
SrG2 (Luig and Watson, 1965); Srrl1 (Rondon et al., 1966); Srmq1 (Berg et al., 1963); SrPs1 and SrMn1 (Sanghi and Baker, 1972); R1 (Loegering and Powers, 1962).
Chromosome Location
1D (Sears et al., 1957; Baker et al., 1970; Anderson et al., 1971); 1DL (Williams and Maan, 1973).
Low Infection Type
; to ;2=.
Environmental Variability
Low.
Origin
Sr18 is present in a very high proportion of common wheat lines (Baker et al., 1970).
Pathogenic Variability
Avirulence in P. graminis f. sp. tritici is rare (Roelfs and McVey, 1979; Luig, 1983). However, avirulence for this gene is widespread in collections of P. graminis f. sp. secalis and in hybrids between P. g. tritici and P. g. secalis (Luig and Watson, 1972).
Reference Stocks
i: I Hope 1D-Ra (Loegering and Harmon, 1969); Sr18/8*LMPG (Knott, 1990); Mq-A and R1-A (Anderson et al., 1971).
s: Chinese Spring*6/Hope 1D (Sears et al., 1957).
v: Mona (Sanghi and Baker, 1972); Pusa (Sanghi and Baker, 1972). Hope Sr2 Sr7b Sr9d Sr17 (Luig, 1983). Reliance Sr5 Sr16 Sr20 (Anderson et al., 1971). Marquis Sr7b Sr19 Sr20 (Anderson et al., 1971). Gabo Sr11 (Luig and Watson, 1965).
Source Stocks
This gene is very difficult to identify with certainty because pathogen isolates avirulent for Sr18 usually possess additional genes for avirulence which render them unable to attack most wheat cultivars. Nevertheless, workers at The University of Sydney demonstrated its presence in most wheats (Baker et al., 1970). Indeed McIntosh (1988a) considered it more convenient to list wheats not possessing Sr18. These included Chinese Spring (Loegering and Harmon, 1969); Eureka (Baker et al., 1970); Federation (Baker et al., 1970); Little Club (Loegering and Harmon, 1969); Morocco (Baker et al., 1970); Prelude (Loegering and Harmon, 1969) and Yalta (Baker et al., 1970) as well as W2691 and Line E, both of which were especially bred at The University of Sydney for susceptibility to pathogen cultures with unusual genes for avirulence, including P18. The presence of p18 in P. graminis f. sp. tritici would be essential because Sr18 occurs in most common wheats. In contrast, P18 is unnecessary in P. graminis f. sp. secalis due to the absence of Sr18 in cereal rye. In this way genes such as Sr18 play a role in host specialisation.
Use in Agriculture
None.
Sr19
(Anderson et al., 1971)
Synonym
Srmq2 (Berg et al., 1963).
Chromosome Location
2B (Anderson et al., 1971); 2BS (Williams and Maan, 1973).
Low Infection Type
1– to 1+ (Roelfs and McVey, 1979), 2C (Huerta-Espino, 1992).
Environmental Variability
Not known.
Origin
Common wheat cv. Marquis. Other sources of Sr19 have not been identified.
Pathogenic Variability
Most collections of P. graminis f. sp tritici are virulent (Roelfs and McVey, 1979; Luig, 1983). Huerta-Espino (1992) recorded two cultures producing IT 2C on lines with Sr19. One of these was collected in the Malagasy Republic and the other in Chile.
Reference Stocks
v: Mq-B (Anderson et al., 1971). Marquis Sr7b Sr18 Sr20 (Anderson et al., 1971).
Source Stocks
None known.
Use in Agriculture
None.
Plate
Not available.
Sr20
(Anderson et al., 1971)
Synonyms
Srmq3 (Berg et al., 1963); Srr13 (Rondon et al., 1966); R2 (Loegering and Powers, 1962).
Chromosome Location
2B (Anderson et al., 1971).
Low Infection Type
2= to 3 (Anderson et al., 1971; Roelfs and McVey, 1979; Roelfs et al., 1992).
Environmental Variability
Not known.
Origin
Common wheat cv. Marquis.
Pathogenic Variability
Most collections of P. graminis f. sp tritici are virulent (Roelfs and McVey, 1979; Huerta-Espino, 1992).
Reference Stocks
v: Mq-C (Anderson et al., 1971); RI-C (Anderson et al., 1971). Marquis Sr7b Sr18 Sr19 (Anderson et al., 1971).
Source Stock
Reliance Sr5 Sr16 Sr18 (Anderson et al., 1971).
Use in Agriculture
None.
Plate
Not available.
Sr21
(The, 1973a) (Plate 3-22)
Chromosome Location
2A (The, 1973a); 2AL (The et al., 1979). Sr21 mapped 2 cM from the centromere and 48 cM from Pm4.
Low Infection Type
; to 23–. The low infection type becomes higher with increasing levels of ploidy (The, 1973a).
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
T. monococcum accessions, including Einkorn C.I.2433 which was adopted by Stakman et al. (1962) as a pathotype differential (The, 1973a). A gene with identical specificity in chromosome 1D of T. tauschii (McIntosh, 1981) is sometimes referred to as SrX (D The, unpublished 1992).
Pathogenic Variability
Polymorphism occurs in most geographic areas. The frequencies of virulence in North America (Roelfs and McVey, 1979) and South America (Huerta-Espino, 1992) are very high compared to other regions. Virulent mutants have been detected in Australian field surveys but none has become established as a common field pathotype (Zwer et al., 1992).
Reference Stocks
i: Sr21/8*LMPG (Knott, 1990).
v: W3586 = Glossy Huguenot/Einkorn//unknown hexaploid (The, 1973a); R.L.5406 = Tetra Canthatch/T. tauschii R.L.5289 (McIntosh, 1981).
dv: Einkorn C.I.2433.
Source Stocks
i: Sr21/5*Aroona; Sr21/5*Condor; Sr21/5*Egret; Sr21/5*Halberd; Sr21/5*Lance; Sr21/5*Oxley; Sr21/5*Teal(The et al., 1988).
v: Hexaploid derivatives of T. monococcum produced by The (1973b). Hexaploid derivatives of T. tauschii possessing SrX (see Origin).
tv: Tetraploid derivatives of T. monococcum (The, 1973b).
dv: T. monococcum accessions (The, 1973a, 1973b). T. tauschii accessions including R.L.5289 (McIntosh, 1981; RA McIntosh, unpublished 1991).
Use in Agriculture
This gene has potential for limited use in areas with low frequencies of virulence if deployed together with other genes. If used alone, rapid increases in the frequency of virulent pathotypes can be anticipated. In addition, current lines with Sr21 are not adequately resistant to avirulent pathotypes as adult plants. The et al. (1988) showed that, in the absence of disease, a number of backcross-derived lines possessing Sr21 gave significantly lower yields than near-isogenic counterparts that did not carry the gene.
Sr22
Kerber, E.R., and P.L. Dyck. “Inheritance of stem rust resistance transferred from diploid wheat (Triticum monocooum) to tetraploid and exaploid wheat and chromosome location of the gene involved.” Canadian Journal of Genetics and Cytology 15: 397-409.
Chromosome Location
7A (Kerber and Dyck, 1973); 7AL . Sr22 is located 30 cM from the centromere, 2 cM from cn-A1 (chlorina phenotype) and more than 50 cM proximal to Pm1/Lr20/Sr15 (The and McIntosh, 1975).
Low Infection Type
; (in diploid wheat) to 2– (in hexaploid wheat) (Kerber and Dyck, 1973); 1– to 2 (Huerta-Espino, 1992).
Environmental Variability
Temperature sensitive; more effective at lower temperatures.
Origin
T. monococcum R.L.5244 (Kerber and Dyck, 1973; The, 1973a). The (1973b) found that Sr22 was present in a range of wild einkorn wheats.
Pathogenic Variability
Virulent cultures were not detected in surveys in Mexico (Singh, 1991), USA (Roelfs and McVey, 1979), South Africa (Le Roux and Rijkenberg, 1987a) and Australia (Zwer et al., 1992). Huerta-Espino (1992) also failed to detect virulence in his international survey. However, Gerechter-Amitai et al. (1971) reported virulence in ten of 12 pathotypes used in Israel.
Reference Stocks
i: Sr22/9*LMPG (Knott, 1990).
v: Chinese Spring/3/Steinwedel*2//Spelmar/T. boeoticum G-21 (The, 1973a); Marquis*5//Stewart*3/T. monococcum R.L.5244 (Kerber and Dyck, 1973; The, 1973a); Steinwedel*2//Spelmar2*/T. boeoticum G-21 (The, 1973a).
tv: Spelmar*2/T. boeoticum G-21 (Gerechter-Amitai et al., 1971; The, 1973a); Stewart*6/ T. monococcum R.L.5244 (Kerber and Dyck, 1973).
dv: Various T. monococcum accessions including G-21 and R.L.5244 (Kerber and Dyck, 1973; The, 1973a, 1973b).
Source Stocks
Australia: BT-Schomburgk; Schomburgk.
Use in Agriculture
Cultivar Schomburgk was released in Australia in 1986. This and a boron-tolerant derivative, BT-Schomburgk are the only commercial cultivars with this gene. Lines carrying Sr22 were reported to be moderately susceptible in the field by Roelfs and McVey (1979).
Sr23
(McIntosh and Luig, 1973 b) (Plate 3-24)
Chromosome Location
2BS (RA McIntosh, unpublished 1978). This gene is completely associated with Lr16. It was initially believed to be located in chromosome 4B, but a Rescue monosomic series was used to establish the location and Rescue carries a 2B-4B reciprocal translocation relative to Chinese Spring (RA McIntosh, unpublished 1980; EDP Whelan, pers. comm. 1982). Sr23/Lr16 are genetically independent of, and presumably distal to, Sr36 (RA McIntosh, unpublished 1980).
Low Infection Type
l++N to 3+CN.
Environmental Variability
Sr23 is expressed only under conditions of high temperature and high light intensity (Luig, 1983). The low infection type is often characterised only by necrosis surrounding some of the otherwise fully compatible uredinia. Roelfs and McVey (1979) observed slight increases in sporulating area with increasing temperature.
Origin
Sr23 was first noted and characterised in common wheat cultivars Selkirk, Exchange and Warden.
Pathogenic Variability
A necrotic response and low infection type with the old Australian pathotype 126-5, 6, 7, 11 is characteristic of plants with this gene. With other pathotypes some necrosis is expressed in otherwise large ‘compatible’ uredinia under high light and temperature conditions. Huerta-Espino (1992) used Exchange as the host tester for Sr23 but it apparently also carries the uncatalogued gene SrMcN. Despite the statement that “all isolates were virulent to …Sr23 and SrMcN” (Huerta-Espino, 1992), three cultures, one each from Burundi, Turkey and Nepal, were recorded as producing ITs 2C to 23C on seedlings of Exchange. These responses seemed to be too high to be conferred by SrMcN (2–; Roelfs and McVey, 1979), but could be associated with Sr23.
Reference Stocks
v: Exchange (McIntosh and Luig, 1973b). Selkirk Sr2 Sr7b Sr9d Sr17 (McIntosh and Luig, 1973b).
Source Stocks
Warden (McIntosh and Luig, 1973b). Etoile de Choisy Sr29 (McIntosh and Luig, 1973b). All stocks carrying Lr16 are assumed to carry Sr23 (see Lr16).
Use in Agriculture
None. Sr23 is not expected to provide significant levels of protection when deployed in susceptible backgrounds (McIntosh and Luig, 1973b).
Sr24
(McIntosh et al., 1976) (Plate 3-25)
Chromosome Location
3D (McIntosh et al., 1976; Smith et al., 1968; Sears, 1973); 3DL (Hart et al., 1976); 3Ag (Sears, 1977). Two other translocation lines produced by Sears involve chromosome 3BL (Sears, 1977). A further translocation is present in cultivar Amigo (see Lr24). Sr24 is completely associated with Lr24 (McIntosh et al., 1976).
Low Infection Type
l– to 22+.
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
Th. ponticum.
Pathogenic Variability
Virulence for Sr24 has been reported in South Africa (Le Roux and Rijkenberg, 1987b) and India (Bhardwaj et al., 1990). Sydney University culture 57096 [pt. 34-(4), 7] produces IT 3 on seedlings with Sr24. This Australian culture is assumed to have arisen from somatic hybridisation between P. graminis f. sp. tritici and P. graminis f. sp. secalis (Luig, 1983). Huerta-Espino (1992) did not find virulence for Sr24 among a wide range of international collections.
Reference Stocks
i: Sr24/9*LMPG (Knott, 1990); various 3D/Ag translocation lines produced by Sears (1972b, 1973, 1977) can be considered near-isogenic to Chinese Spring.
s: Chinese Spring 3Ag(3D) (Sears, 1973).
v: Agent (McIntosh et al., 1976).
[[{“attributes”:{},”fields”:{}}]]
Source Stocks
Australia: Janz Sr5; Torres Sr5 (Brennan et al., 1983); Vasco Sr5. Sunco Sr5 Sr6 Sr8a Sr36. Sunbird Sr5 Sr8a. Sunelg Sr26.
North America: Abilene; Amigo (The et al., 1992); Arapahoe; Arkan; Blueboy II (McIntosh et al., 1976); Centura; Century; Cimmaron; Cloud; Cody; Coker 9733; Collin; Fox; Mesa; Norkan; Parker 76; Payne; Rio Blanco; TAM200; Terral 101; Thunderbird; Timpaw; Trailblazer; Twain; Wanken. Karl Sr2 Sr9d. Siouxland Sr5 Sr31 (Schmidt et al., 1985). Butte 86 Sr6 (Modawi et al., 1985). Colt Sr6 Sr8a Sr9a Sr17. Osage Sr17; Sage Sr17 (Livers, 1978). Jasper Sr31; Longhorn Sr31.
South Africa: Gamka; Karee; Kinko; Palmiet; SST25; SST44 = T4R; SST102; Wilge (Le Roux and Rijkenberg, 1987b); SST23 (Sharma and Gill, 1983).
Use in Agriculture
Derivatives of Agent were widely used in the USA and South Africa as a source of leaf rust resistance. P. graminis pathotypes with virulence for Sr24 were found in South Africa in 1984 (Le Roux, 1985) and in India in 1989 (Bhardwaj et al., 1990). All backcross derivatives with Lr24/Sr24 from Agent added to white-seeded Australian wheats were found to be red seeded (RA McIntosh, unpublished 1973). Several of Sears’s 3D/Ag translocation stocks were then used, and of these, transfers #3 and #14 gave white-seeded derivatives. These lines were used to develop Australian cultivars, the first of which, Torres, was released in 1983. Australian lines were subsequently used in Indian backcrossing programs but virulence for Sr24 was detected before cultivars could be commercialised.
The et al. (1992) found that Amigo carried Sr24/Lr24. Amigo carries an independent translocation with stem rust resistance from rye and, although the chromosome location of Sr24 in this red-seeded stock was unknown, white-seeded derivatives were easily selected in Australian backcrossing programs.
Sr25
(McIntosh et al., 1976) (Plate 3-26)
Chromosome Location
7D (Sharma and Knott, 1966). 7DL (McIntosh et al., 1976; Dvorak and Knott, 1977); 7AL (Eizenga, 1987); 7Ag (Sears, 1973). In most stocks Sr25 is associated with Lr19.
Low Infection Type
1– to 23. Low infection types recorded by Knott (1980, 1990) were generally lower than those obtained in Australia.
Environmental Variability
Low (Roelfs and McVey, 1979), but probably inadequately researched (Luig, 1983). Gough and Merkle (1971) suggested that lines with Sr25 may become more susceptible at high temperatures.
Origin
Th. ponticum.
Pathogenic Variability
Luig (1983) mentioned an Israeli isolate with putative virulence. Huerta-Espino (1992) identified one virulent culture from Ethiopia and two virulent cultures from Nepal.
Reference Stocks
i: Sr25/9*LMPG (Knott, 1990). Sears’ independently derived 7D/Ag translocation stocks (Sears, 1973) can be considered near-isogenic to Chinese Spring. Agatha Sr5 Sr9g Sr12 Sr16 = T4 (Sharma and Knott, 1966) is near-isogenic to Thatcher.
su: Chinese Spring 7Ag(7D) (Sears, 1973). Agrus, a 7Ag(7D) stock (Sharma and Knott, 1966).
Source Stocks
Because of its close association with Lr19 (McIntosh et al., 1976), Sr25 should be present in Oasis F86, Indis and Sunnan (see Lr19).
Use in Agriculture
Lines of Chinese Spring with Lr19/Sr25 can become moderately rusted with avirulent cultures in breeding nurseries and losses may occur (McIntosh et al., 1976; Roelfs and McVey, 1979). The use of this gene in breeding can be justified by its linkage with Lr1 9. However, lines with Sr25/Lr19 from Agatha and Sears’ translocations are characterised by high levels of yellow pigment in the endosperm. Knott (1980) obtained two mutants of Agatha with reduced levels of yellow pigment in the flour. One of these mutants lacked Sr25. Marais (1992a) reported that a gene very similar to Sr25, and designated Sr25d, was present in the Inia 66 x Th. distichum derivative, Indis. Marais (1992a, 1992b) also obtained mutants with reduced yellow pigment in Indis derivatives and some of these lacked Sr25d.
Sr26
(McIntosh et al., 1976) (Plate 3-27)
Chromosome Location
6A (6A/6Ag translocation) (Knott, 1961); 6AL (J Fisher, pers. comm. 1975).
Low Infection Type
0; to 2–.
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
Th. ponticum
Pathogenic Variability
Virulence has not been confirmed in field isolates (Luig, 1983; Huerta-Espino, 1992).
Reference Stocks
i: Sr26/9*LMPG (Knott, 1990).
s: Chinese Spring 6Ag and 6AgL substitutions for 6A, 6B and 6D, respectively (D The, unpublished 1990).
Source Stocks
Australia: Avocet (J Fisher, pers. comm. 1979); Flinders (Syme, 1983); Harrier; Kite (Luig, 1983); Takari (Fletcher, 1983). Eagle Sr9g (McIntosh ex al., 1976). Sunelg Sr24. Bass Sr36 (Syme et al., 1983).
Use in Agriculture
Sr26 has been used as a source of resistance only in Australia where the first cultivar, Eagle, was released in 1971. The et al. (1988) showed that backcross-derived lines with Sr26 yielded 9% less than sr26 sibs. However, cultivars Flinders, Harrier, Kite, Takari, Eagle and Sunelg were widely grown and competed satisfactorily with contemporary cultivars.
Sr27
(McIntosh, 1988a) (Plate 3-28)
Chromosome Location
3A (3A.3R translocation) (Acosta, 1962).
Low Infection Type
0; to 12=.
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
S. cereale cv. Imperial.
Pathogenic Variability
Virulence for Sr27 is rare. Harder et al. (1972) isolated an east African culture virulent on a Pembina line with Sr27. Luig (1983) reviewed Australian work showing that cultures of P. graminis f. sp. secalis and certain hybrids of P. graminis f. sp. tritici and P. graminis f. sp. secalis were virulent. McIntosh et al. (1983) showed that isolates of P. graminis f. sp. tritici from triticale cv. Coorong were virulent on wheat seedlings with Sr27. A greenhouse mutant with virulence on Coorong was also virulent on seedlings with Sr27. The results were accepted as evidence that the resistance gene in Coorong and many other triticale lines developed in Mexico was Sr27. Virulence on triticale cultivars with Sr27 was found in South Africa in 1988 (Smith and Le Roux, 1992).
Reference Stocks
i: Chinese Spring WRT 238.5 (Acosta, 1962). Justin, Selkirk and Pembina derivatives of WRT 238.5 (Stewart et al., 1968). Sr27/9*LMPG (Knott, 1990).
ad: Chinese Spring + Imperial 3R (2n = 44) (ER Sears, pers. comm. 1969).
Source Stocks
Widespread in triticales, for example Coorong, Towan, Dua, Tyalla, Arabian, Bura S (McIntosh et al., 1983). Some sources of Setter carry Sr27.
Use in Agriculture
Wheats with Sr27 have not been released in agriculture. Pathogen samples collected from Coorong triticale in eastern Australia were shown to be virulent for Sr27 (McIntosh et al., 1983). Coorong and many other triticale lines were extremely susceptible to this pathotype. McIntosh et al. (1983) further showed that Sr27 occurred at high frequency in lines present in nurseries distributed from CIMMYT and gave warning of genetic vulnerability in triticale. Cultivar Satu was recommended in Australia as a replacement for Coorong, but a further mutant of the ‘Coorong’ pathotype quickly developed. Genetic studies indicated that a single gene in Satu was allelic with the gene (Sr27) in Coorong.
Sr28
(McIntosh, 1978) (Plate 3-29)
Synonym
SrKtal (Berg et al., 1963).
Chromosome Location
2BL (McIntosh, 1978). Sr28 was located 18 cM distal to Sr9 and 33 cM proximal to Sr16.
Low Infection Type
0; to 2=.
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
Common wheat cv. Kota. Kota was included in the differential set of Stakman et al. (1962).
Pathogenic Variability
Virulence is common in all geographic areas (Luig, 1983). Huerta-Espino (1992) found significant levels of avirulence among cultures from Ethiopia and Nepal; otherwise virulence levels were high.
Reference Stocks
i: Line AD = W2691*5/Kota (McIntosh, 1978).
s: Ceres Sr7b (McIntosh, 1978); Kota Sr7b (McIntosh, 1978).
Source Stocks
None.
Use in Agriculture
Sr28 is of limited use in current wheat breeding. However, it was the basis for resistance in cultivar Ceres released in the USA and rendered susceptible by race 56 in 1935.
Sr29
(Dyck and Kerber, 1977b) (Plate 3-30)
Synonym
SrEC (McIntosh et al., 1974).
Chromosome Location
6DL (Dyck and Kerber, 1977b); 6DS (Zeller and Oppitz, 1977); Sr29 appeared to be genetically independent of the centromere (Dyck and Kerber, 1977b).
Low Infection Type
1 to 3.
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
Common wheat. Sr29 appears to be a gene of European origin.
Pathogenic Variability
Roelfs and McVey (1979) reported that a few avirulent cultures gave low infection types that were slightly higher than usual. Huerta-Espino (1992) reported virulence only among cultures from western Asia, eastern Europe, Egypt, Ethiopia and Turkey.
Reference Stocks
i: Prelude/8*Marquis//Etoile de Choisy (Dyck and Kerber, 1977b).
v: Etoile de Choisy W3550 Sr23 (McIntosh et al., 1974).
Source Stocks
Hela; Mara; Slavia; Vala (Bartos and Stuchlikova, 1986). Moisson Sr23 (Luig, 1983).
Use in Agriculture
Adult plants with this gene are moderately susceptible (Roelfs and McVey, 1979). The gene has been used for stem rust control in European wheats, but has not been incorporated into North American winter wheats or spring wheats grown outside of Europe.
Sr30
(Knott and McIntosh, 1978) (Plate 3-31)
Chromosome Location
5DL (Knott and McIntosh, 1978). Sr30 is genetically independent of Pm2 (in 5DS) and Lr1 (Knott and McIntosh, 1978).
Low Infection Type
1+ to 3. Some lines with Sr30 produce lower responses than others (Roelfs and McVey, 1979; Knott, 1990).
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
Common wheat cv. Webster which was introduced to the USA from Russia.
Pathogenic Variability
Luig (1983) reported that Sr30 was generally effective in North America and Europe, although Roelfs and McVey (1979) mentioned that race 11-RHR was virulent. In Australia, several virulent pathotypes became prevalent after 1968 (Luig, 1983). Virulence was reported as frequent in South Africa (Le Roux and Rijkenberg, 1987a). Huerta-Espino (1992) found virulence in a number of countries with moderate to high levels among samples from Spain, Ethiopia, Turkey, Pakistan and a number of South American countries.
Reference Stocks
i: Sr30/7*LMPG-1; Sr30/7*LMPG-2; Sr30/7*LMPG-3 (Knott, 1990).
v: Festiguay W2706 (Knott and McIntosh, 1978); Webster W973 (Knott and McIntosh, 1978); Mediterranean W1728 (Singh and McIntosh, 1985). Klein Cometa Sr8b (Singh and McIntosh, 1986a).
Source Stocks
Work at The University of Sydney Plant Breeding Institute has demonstrated the presence of Sr30 in several Mexican wheats and Australian derivatives.
Australia: Dollarbird Sr2 Sr8a Sr9g. Hartog (= Pavon ‘S’) Sr2 Sr8a Sr9g Sr12. Batavia Sr2 (heterogeneous) Sr8a Sr12. Houtman Sr2 Sr9gSr17. Rosella Sr5 Sr7b Sr8a Sr12. Lark Sr5 Sr8a. Sunfield Sr5 Sr8a Sr9b Sr12. Osprey Sr5 Sr8a Sr12. Katunga Sr8a. Banks Sr8a Sr9b Sr12; Vulcan Sr8a Sr9b Sr12. Sunstar Sr8a Sr9e Sr12. Lilimur Sr8a Sr17 (heterogeneous). Cranbrook (= Flicker ‘S’)Sr8a Sr9g Sr12 Sr17.
CIMMYT: Lerma Rojo 64A.
Use in Agriculture
Because of the absence of virulence, Webster was once considered to be almost universally resistant (Hart, 19.31). However, when the resistance was deployed in the Australian cultivar Festiguay, virulent pathotypes increased on this cultivar. These pathotypes declined after Festiguay was withdrawn from cultivation. More recently, a distinctive virulent pathotype was isolated in eastern Australia (Park and Wellings, 1992). Although this pathotype can overcome the resistance of some current wheats with Sr30, it has remained at extremely low levels.
Sr31
(McIntosh, 1988a) (Plate 3-32)
Chromosome Location
1BS (1BL.1RS translocations) or 1R(1B) substitutions (Mettin et al., 1973; Zeller, 1973). Some wheat cultivars comprise both substitution and translocation biotypes (Zeller, 1973).
Low Infection Type
l– to 2.
Environmental Variability
None reported.
Origin
S. cereale cv. Petkus. Most wheats with Sr31 were derived from wheat x rye hybrid derivatives produced in Germany in the 1930s (see Mettin et al., 1973; Zeller, 1973).
Pathogenic Variability
Huerta-Espino (1992) recorded a virulent culture in a collection from Turkey.
Reference Stocks
i: Federation*4/Kavkaz (RA McIntosh and CR Wellings, unpublished 1992); Thatcher*6/ ST-1.25, R.L.6078 (PL Dyck, pers. comm. 1986).
v: Aurora (Zeller, 1973); Kavkaz (Zeller, 1973).
Source Stocks
All wheats with Lr26 and Yr9 (see Lr26, Yr9). Sr31 is present in many European wheats and some Chinese and USA wheats as well as being widely used in wheats distributed by the CIMMYT program (e.g. Bobwhite and Veery selections) and continues to occur at high frequencies in CIMMYT breeding populations.
Australia: Grebe; Warbler.
China: Feng-Kang 2; Feng-Kang 8; Jan 7770-4; Jin-Dan 106; Lu-Mai 1; Yi 78-4078. Dong Xie 3 Sr5; Dong Xie 4 Sr5. See Hu and Roelfs (1986).
CIMMYT: Alondra; Angostura 88; Bacanora 81; Bobwhite S; Cumpas 88; Curinda 87; Genaro 81 (=Veery#3); Glennson 81 (=Veery #1); Guasave 81; Mochis 88; Seri 82 (Veery #5); Ures 81 (= Veery #2). See Singh and Rajaram (1991) and Singh (1993). Many of these wheats or sibs are grown in other countries (Villareal and Rajaram, 1988).
Europe: Aurora; Benno; Bezostaya 2; Burgas 2; Clement; Kavkaz; Lovrin 10; Lovrin 13; Mildress; Neuzucht; Skorospelka 35; Weique; Zorba.
Indian Subcontinent: CPAN 1922; HUW 206; Pakistan 81; Sarhad 82. See Singh and Gupta (1991).
South Africa: Gamtoos (= Veery #3).
USA: Excel; Freedom; Salmon (USA). Siouxland Sr5 Sr24. Longhorn Sr24.
Use in Agriculture
The value of Sr3l as a source of protection against stem rust is difficult to determine. The widespread international distribution of wheats with Sr31 may reflect the broad agronomic adaptability of these materials rather than the unique contribution of stem rust resistance. Despite successful use in many areas, wheats with Sr31 have not been widely grown in Australia due to potential problems in bread-making. The only cultivars registered are Grebe, an Egret derivative used for biscuit (cookie) quality flour, and Warbler, a feed wheat. Other wheat derivatives with 1RS have stem rust resistance characterised by low infection types similar to those produced by lines with Sr3l. KW Shepherd and coworkers produced 1D.1RS (Koebner and Shepherd, 1986) and 1B. 1RS derivatives of Imperial rye, whereas The et al. (1992) described a gene presumably associated with 1AL.1RS in Amigo. No cultivar with 1RS from Imperial has been produced but several derivatives of Amigo are grown in the USA. The 1AL.1RS chromosome carries the gene Gb5 for resistance to greenbug (Schizaphis graminum). It is not known if the gene from Imperial rye and the gene in Amigo are the same as Sr31. The presence of Sr31 in wheat is readily confirmed by the concurrent presence of Lr26 and Yr9 (see respective sections) as well as by cytological and biochemical methods. These were reviewed or described by Javornik et al. (1991), May and Wray (1991), Gupta and Shepherd (1992).
Sr32
(McIntosh, 1988a) (Plate 3-33)
Chromosome Location
Independent translocations located in chromosomes 2A (RA McIntosh, unpublished 1974), 2B (ER Sears, pers. comm. 1982) and 2D (ER Sears, pers. comm. 1982).
Low Infection Type
l+ to 2C.
Environmental Variability
None reported.
Origin
T. speltoides.
Pathogenic Variability
The international survey of Huerta-Espino (1992) as well as parhogenicity surveys in the USA (Roelfs et al., 1991), Canada (Harder and Dunsmore, 1990), Mexico (Singh, 1991), South Africa (Le Roux and Rijkenberg, 1987a) and Australia (RF Park, unpublished 1992) failed to find virulence.
Reference Stocks
i: W3531, a Chinese Spring stock produced by ER Sears and involving a translocation to chromosome 2A (RA McIntosh, unpublished 1974). This stock is characterised by having adhering glume fragments, especially in the crease region of the grain. This primitive feature tends to be associated with the absence of at least a part of wheat chromosome 2A. C77.19, a CS/T. speltoides derivative, is a cleaner threshing line with Sr32 present in chromosme 2B. Sears later produced four further transfers with Sr32 present in chromosomes 2B and 2D. These are accessioned in The University of Sydney cytogenetics collection as C82.1 (chromosome 2B), C82.2 (2D), C82.3 (2D) and C82.4 (2D). The lines designated CS Sr32 (Le Roux and Rijkenberg, 1987b) and ER 5155 (Roelfs and Martens, 1988) probably correspond to W3531 or C77.19.
Source Stocks
Australian backcross lines produced at The University of Sydney Plant Breeding Institute.
Use in Agriculture
Early breeding studies at The University of Sydney using W3531 failed to separate resistance from the adherent glume phenotype. Backcross derivatives with Sr32 derived from C77. 19 were produced and distributed to wheat breeders but no line was commercialised or used in further breeding. The reasons for this are unknown. Sears (pers. comm. 1982) suggested that C82.2 was the most normal of the translocation lines.
Sr33
(McIntosh, 1988a) (Plate 3-34)
Synonym
SrSQ (Kerber and Dyck, 1979).
Chromosome Location
1DL (Kerber and Dyck, 1979). Because of linkage with Lr21, Rg2 and Gli-D1 (Jones et al., 1990), Sr33 must be located in 1DS. Czarnecki and Lukow (1992) mapped it 9 cM from Gli-D1 whereas Jones et al. (1991) obtained estimates of 5.6 and 7.6 cM for the same interval. Sr33 was proximal to Gli-D1.
Low Infection Type
;1– in diploid stocks, 2 in hexaploid derivatives (Kerber and Dyck, 1979). Huerta-Espino (1992) recorded ITs 1–C to 2.
Environmental Variability
None reported.
Origin
T. tauschii R.L.5288.
Pathogenic Variability
Virulence has not been reported, but lines with Sr33 have not been tested widely. Pathogenicity surveys in the USA (Roelfs et al., 1991), Mexico (Singh, 1991) and Australia as well as the study of Huerta-Espino (1992) failed to detect virulence for Sr33.
Reference Stocks
v: R.L.5405 = C78.15 = Tetra Canthatch/T. tauschii R.L.5288 (Kerber and Dyck, 1979).
dv: T. tauschii R.L.5288 (Kerber and Dyck, 1979).
Source Stocks
Several Australian backcross derivatives produced at The University of Sydney Plant Breeding Institute.
Use in Agriculture
No wheat with Sr33 has been commercialised. Kerber and Dyck (1979) reported that Sr33 was closely linked in repulsion with Lr21. RA McIntosh (unpublished, 1981) tested F3 lines of the Sr33/Lr21 cross produced by ER Kerber, and demonstrated the proximity of Sr33 to both Lr21 and a stem rust resistance gene with the same specificity as Sr21 and present in the Lr21 stock. Although he believed that recombination of Sr33 and Sr21 had occurred he was unable to confirm this because of possible meiotic instability in the amphiploid materials being examined. The study is being repeated using backcross derivatives. Preliminary results indicate a genetic distance of 4 cM between Sr33 and the Sr21 -like gene.
Sr34
(McIntosh et al., 1982) (Plate 3-35)
Chromosome Location
2A (2A/2M, translocation), 2D (2D/2M translocation), 2M (McIntosh et al., 1982). Sr34 is present in wheats with Yr8.
Low Infection Type
1N to 3. 2C to 23CN (Huerta-Espino, 1992).
Environmental Variability
Most effective at low temperatures.
Origin
T. comosum.
Pathogenic Variability
Avirulence for Sr34 is relatively rare in Australia with only two older related pathotypes producing low ITs on tester stocks (McIntosh et al., 1982). Knott (1990) reported avirulence in a Canadian culture of race 111 which is known to be widely avirulent. In the survey of cultures carried out by Huerta-Espino (1992) avirulence was common with levels approaching 50% in south Asia (Pakistan and Nepal), Ethiopia, Kenya, the Malagasy Republic and South America. He found no virulence among collections from China but he cited unpublished 1984 results from CC Hu and AP Roelfs indicating a virulence level of 84%. Thus for international comparisons of pathogenicity, a line with Sr34 would be a useful differential.
Reference Stocks
i: Sr.34/6*LMPG (Knott, 1990); Compair; Chinese Spring 2D/2M 3/8 (C77.1); Chinese Spring 2A/2M 4/2 (C77.2) (McIntosh et al., 1982).
su: Chinese Spring 2M(2A) (McIntosh et al., 1982).
Source Stocks
None.
Use in Agriculture
Translocations with Sr34 have not been deployed in commercial cultivars.
Sr35
(McIntosh et al., 1984) (Plate 3-36)
Synonym
SrTm1 (Valkoun et al., 1986).
Chromosome Location
3AL (McIntosh et al., 1984). Sr35 was located 41.5 cM from the centromere and showed 1% recombination with R2 derived from the same source.
Low Infection Type
0; to ;.
Environmental Variability
None reported.
Origin
T. monococcum. Sr35 is present in a selection from The University of Sydney accession C69.69 (P.I.264935) and in the Canadian accession G2919 (McIntosh et al., 1984). It was independently transferred from these accessions to tetraploid and hexaploid wheats in Canada and to hexaploid wheat in Australia.
Pathogenic Variability
Sr35 is effective against most Australian pathotypes except cultures of standard race 126 and a mutant culture (82-L-2) isolated during studies in the greenhouse. Many North American cultures are avirulent (McIntosh et al., 1984). In the survey of Huerta-Espino (1992) virulence was found in Ethiopia, Kenya, the Malagasy Republic, Nepal, China, Argentina, Brazil and Chile.
Reference Stocks
i: C81.42 = R.L.6071*7/G2919 Sr9e (McIntosh et al., 1984).
v: M80.3990, M80.4635, M80.4636 derived from C69.69 (McIntosh et al., 1984). Backcross derivatives of cvv. Zlatka and Yubinlenaya (Valkoun et al., 1986).
tv: Stewart*8/G2919 Sr9e (McIntosh et al., 1984).
dv: Components of C69.69 = P.I.264935; G2919 (McIntosh et al., 1984).
Source Stocks
Backcross derivatives produced at The University of Sydney, for example Sr35/3*77W549, Sr35/4*Canna.
Use in Agriculture
Although not yet exploited, Sr35 should have a role if used in gene combinations. White-seeded derivatives with Sr35 were recovered in the genetic studies conducted by McIntosh et al. (1984).
Sr36
(McIntosh, 1988a) (Plate 3-37)
Synonym
SrTt1 (McIntosh and Gyarfas, 1971).
Chromosome Location
2B (Nyquist, 1957); 2BS (Gyarfas, 1978). Stem rust and powdery mildew resistances in the wheats C.I. 12632 and C.I. 12633 were originally thought to be determined by duplicate linked factors (Allard and Shands, 1954) but Nyquist (1962) demonstrated that a single gene was involved with differential fertilisation resulting in variable ratios. The mildew resistance factor was subsequently designated Pm6 (Jorgensen and Jensen, 1973). In early studies at The University of Sydney, Sr36 behaved as an allele of Sr9. McIntosh and Luig (1973a) reported a rare recombinant combining Sr36 and Sr9e. The line was later designated Combination III (Luig, 1983). In further genetic studies involving this stock, Sr36 was normally transmitted and showed about 20% recombination with Sr9 (McIntosh and Luig, 1973a). Sr36 recombined with Sr9b but not with Lr23, located in chromosome 2BS. In a separate study with progeny of a monosomic 2B line derived from Combination III, chromosome misdivision products that separately carried Sr9e and Sr36 were obtained (Gyarfas, 1978). This demonstrated that the genes wete located in different chromosome arms.
Low Infection Type
Usually 0;=, but sometimes X. Apparent heterogenous infection types of 0;3 (Knott, 1990) and 0;4 (Roelfs and McVey, 1979) were reported in North America.
Environmental Variability
Under Australian greenhouse conditions, Sr36 appears to produce higher infection types in winter when both light intensity and temperatures are lower than in summer. AP Roelfs (pers. comm. 1988) observed mixed responses with some cultures in autumn and spring but not in summer or winter.
Origin
T. timopheevii. Independent transfers to hexaploid wheat resulted in C.I. 12632 and C.I. 12633 (Allard and Shands, 1954), Timvera (Pridham, 1939) and C.I.13005 (Atkins, 1967).
Pathogenic Variability
Virulence has arisen on at least two independent occasions in Australia, resulting in the susceptibility of Mengavi in 1961 (Luig and Watson, 1970) and Cook in 1984 (Zwer et al., 1992). A similar experience occurred in South Africa following the deployment of Sr36 in wheat-growing areas. In North America, early isolates of race 15B were characterised by virulence on T. timopheevii and, by inference, wheats such as C.I.12632 and C.I.12633. Later isolates of 15B were virulent on C.I.12632 and avirulent on T. timopheevii. These observations were confirmed by RA McIntosh (unpublished, 1970) working at the University of Missouri. In later studies, wheats with Sr36 were described as having low receptivity to pathotypes normally considered virulent (Rowell and McVey, 1979; Roelfs and McVey, 1979; Rowell 1981a, 1981b, 1982).
Huerta-Espino (1992) found pathogenic variation in most of the major regions from which he obtained samples. This indicated that a line with Sr36 would be a useful worldwide differential.
Reference Stocks
i: Sr36/8*LMPG (Knott, 1990); Line C, a W2691 backcross derivative with Sr36 (Luig, 1983).
v: C.I.12632 (= W1656); C.I.12633 (= W1657); Idaed 59; Mengavi; Timvera (McIntosh and Gyarfas, 1971).
Source Stocks
Australia: Songlen Sr2 Sr5 Sr6 Sr8a. Timgalen Sr5 Sr6. Cook Sr5 Sr6 Sr8a. Mendos Sr7a Sr11 Sr17. Combination III W3486 Sr9e (Luig, 1983).
Mexico: Zaragosa 75 (Le Roux and Rijkenberg, 1987b).
South Africa: Dipka; Flamink; Gouritz; SST101; SST107. See Le Roux and Rijkenberg (1987b) and Sharma and Gill (1983).
USA: Hand; Kenosha; Purdue; Roughrider; Vernum; Wisconsin Supremo. Arthur Sr2 Sr5 Sr8a; Arthur 71 Sr2 Sr5 Sr8a.
Use in Agriculture
Sr36 has been a very valuable gene for wheat production in Australia. It was initially used in cv. Mengavi which was rendered very susceptible by a new pathotype detected very soon after registration. Sr36 was then combined with Sr7a, Sr11 and Sr17 and released in cv. Mendos which in turn succumbed to stem rust after a few years. In 1967 Timgalen was released as a prime hard quality wheat in rust-prone areas. The source of Sr36 in Timgalen is unknown but is presumed to be C.I.12632 or a derivative. Timgalen was followed with the derivatives, Timson, Songlen, Shortim and Cook over subsequent years. Stem rust was found on Cook wheat in 1984 (Zwer et al., 1992). Because of the activities of the National Wheat Rust Control Program, Cook derivatives with additional resistance genes (such as Sunco with Sr24) were released in 1985 and Cook was rapidly withdrawn. The Cook-attacking pathotype then declined to negligible levels, thus providing an enhanced resistance to the Cook derivatives (Park and Wellings, 1992).
According to Roelfs and McVey (1979) and supported by the studies of Rowell (1982) some pathotypes considered virulent on lines with Sr36 developed more slowly and gave lower numbers of pustules on genotypes with Sr36. This contrasted with the Australian experience with Mengavi and Mendos which were seriously affected by the relevant virulent pathotypes.
Sr37
(McIntosh, 1988a) (Plate 3-38)
Synonym
SrTt2 (McIntosh and Gyarfas, 1971).
Chromosome Location
4B (Gyarfas, 1978).
Low Infection Type
0;, ;1, ;3C, depending on pathogen culture (McIntosh and Gyarfas, 1971). Susceptible off-types are relatively common.
Environmental Variability
Low (Roelfs and McVey, 1979).
Origin
T. timopheevii.
Pathogenic Variability
Luig (1983) discussed results of an international survey which indicated that virulence for Sr37 occurred in the USA, Canada, Brazil, Pakistan, India, Italy and Israel. Virulence for Sr37 has not been detected recently in the USA (Roelfs et al., 1990, 1991), or in the regions surveyed by Huerta-Espino (1992).
Reference Stocks
v. Line W = W3563 (McIntosh and Gyarfas, 1971).
tv: Various accessions of T. timopheevii of which W1899 and C.I.11802 should be regarded as the standards (McIntosh and Gyarfas, 1971).
Source Stocks
Other sources of T. timopheevii are listed in McIntosh and Gyarfas (1971).
Use in Agriculture
Sr37 has not been exploited in cultivated durums or common wheat. Although potentially useful for gene combinations from a pathological viewpoint, the T. timopheevii chromosome segment (indeed most of the 4B chromosome) bearing Sr37 has only limited homology with the equivalent chromosome in common wheat (Gyarfas, 1978; Dvorak et al., 1990). Consequently, any common wheat line with Sr37 would have to be derived from a translocation event leading to increased chromosomal homology with wheat chromosome 4B.
McIntosh and Gyarfas (1971) concluded there may be as few as three genes for resistance to stem rust in T. timopheevii, that is, Sr36, Sr37 and possibly a third factor present in the common wheat derivative C.I.13005 (Atkins, 1967). Gyarfas (1978) later presented evidence for a fourth gene which she designated SrTt3 in the common wheat derivative, Line AH (see SrTt3).
Sr38
(Bariana, 1991; Bariana and McIntosh, 1993) (Plate 3-39)
Chromosome Location
2AS. Sr38 is completely linked with Lr37 and Yr17, and is closely linked in repulsion with Lr17 (Bariana and McIntosh, 1993).
Low Infection Type
X, often with very large pustules towards the leaf base.
Environmental Variability
More effective at lower temperatures.
Origin
T. ventricosum.
Pathogenic Variability
Virulence has not been reported, although this gene has not been widely tested.
Reference Stocks
i: Thatcher*8/VPM1, R.L.6081 (PL Dyck, pers. comm. 1991).
v: VPM1 (Doussinault et al., 1981, 1988).
Source Stocks
Australia: Trident (= Spear*4/VPM1). Sunbri Sr5 Sr6 Sr8a Sr36 (Brown et al., 1991). Various backcross-derived lines generated at the PBI, Cobbitty.
UK: Rendezvous (Bariana, 1991).
USA: Hyak (RA McIntosh, unpublished 1990); Madsen (RA McIntosh, unpublished 1990).
Use in Agriculture
Because of the associated resistances to all three rust diseases, wheats with Sr38, Lr37 and Yr17 will be useful in most parts of the world. VPM1 was initially selected as a breeding parent in Australia because of high levels of resistance to all three rusts. The phenomenon of linkage was noted locally during the backcrossing, following tests on Rendezvous bred in the UK and on Hyak and Madsen bred in the Pacific Northwest of the USA, and was demonstrated formally by Bariana and McIntosh (1993). Rendezvous, Hyak and Madsen were bred primarily for resistance to eyespot or strawbreaker disease (caused by Pseudocercosporella herpotrichoides) controlled by gene Pch in chromosome 7DL. Selection for stripe rust resistance, also practiced by the breeders, presumably led to the leaf rust and stem rust resistances present in these cultivars. The Australian cultivar Sunbri was bred for rust resistance and is assumed not to carry Pch.
Sr39
(ER Kerber, pers. comm. 1991) (Plate 3-40)
Chromosome Location
2B (Kerber and Dyck, 1990). Sr39 showed 3% recombination with Lr35 (Kerber and Dyck, 1990) and segregated independently of Sr32 and Lr13 (ER Kerber, pers. comm. 1991).
Low Infection Type
1 to 2. Resistance is incompletely dominant (Kerber and Dyck, 1990).
Environmental Variability
None reported.
Origin
T. speltoides R.L.5344.
Pathogenic Variability
Virulence has not been reported, although wheats with this gene have not been tested widely. An intermediate response was produced with one University of Sydney PBI culture (see Plate 3-33 C).
Reference Stocks
i: Marquis-K*8/R.L5344, R.L.5711 (Kerber and Dyck, 1990).
amphiploid: T. speltoides R.L.5344/T. monococcum R.L.5346, R.L.5347 (Kerber and Dyck, 1990).
Source Stocks
None.
Use in Agriculture
This gene has not been adequately assessed for use in agriculture.
Sr40
(Dyck, 1992) (Plate 3-41)
Synonym
SrA (Dyck, 1992).
Chromosome Location
Probably 2BS (Dyck, 1992). The arm location was based on linkage with other genes in chromosome 2B, that is, 0.01 with Lr13, 0.05 with Lr23, 0.34 with Lr16, 0.22 with Sr36 and 0.28 with Sr9.
Sr41
| Genome Location | 4D | |
| Linked Genes | ||
| Source | Waldron Wheat | |
| Low infection type | Seedling | Adult |
| 23C | ? |
Data courtesy of Tom Fetch
Sr42
| Genome Location | 6DS | |
| Linked Genes | SrCad | |
| Source | Norin 40 | |
| Low infection type | Seedling | Adult |
| 12- | ? |
Data courtesy of Tom Fetch
Sr43
| Genome Location | 7DL | |
| Linked Genes | ||
| Source | Thino ponticum | |
| Low infection type | Seedling | Adult |
Data courtesy of Tom Fetch
Sr44
| Genome Location | 7DS | |
| Linked Genes | ||
| Source | Thino. intermedium | |
| Low infection type | Seedling | Adult |
| ;2 | R |
Data courtesy of Tom Fetch
Sr45
| Genome Location | 1DS | |
| Linked Genes | ||
| Source | T. tauschii | |
| Low infection type | Seedling | Adult |
| 0; |
Data courtesy of Tom Fetch
Sr46
| Genome Location | 2DS | |
| Linked Genes | ||
| Source | Ae. tauschii var meyeri | |
| Low infection type | Seedling | Adult |
Data courtesy of Tom Fetch
Sr47
| Genome Location | 2BL | |
| Linked Genes | ||
| Source | Ae. Speltoides PI 369590 | |
| Low infection type | Seedling | Adult |
Data courtesy of Tom Fetch
Sr48
| Genome Location | 2AL | |
| Linked Genes | ||
| Source | T. aestivum | |
| Low infection type | Seedling | Adult |
| 22+ |
Data courtesy of Tom Fetch
Sr49
| Genome Location | 5BL | |
| Linked Genes | ||
| Source | T. aestivum | |
| Low infection type | Seedling | Adult |
| 2- to 2 |
Data courtesy of Tom Fetch
Sr50
| Genome Location | 1DS | |
| Linked Genes | ||
| Source | Imperial Rye | |
| Low infection type | Seedling | Adult |
Data courtesy of Tom Fetch
Sr51
| Genome Location | 3A, B, D | |
| Linked Genes | ||
| Source | A. searsii | |
| Low infection type | Seedling | Adult |
Data courtesy of Tom Fetch
Sr52
| Genome Location | 6AS | |
| Linked Genes | ||
| Source | Dasypyrum villosum | |
| Low infection type | Seedling | Adult |
| 2- |
Data courtesy of Tom Fetch
Sr53
| Genome Location | 5DL | |
| Linked Genes | ||
| Source | A. geniculata TA5599 | |
| Low infection type | Seedling | Adult |
| 2± |
Data courtesy of Tom Fetch